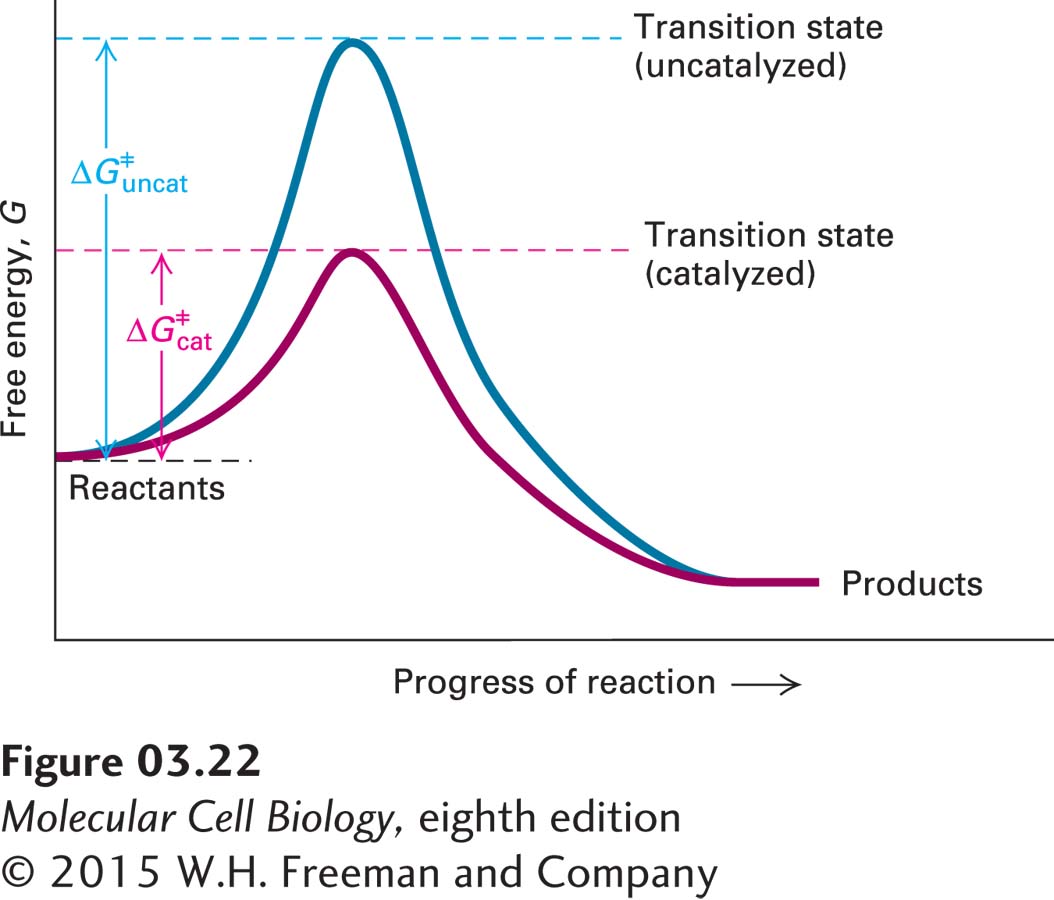
FIGURE 3- h-