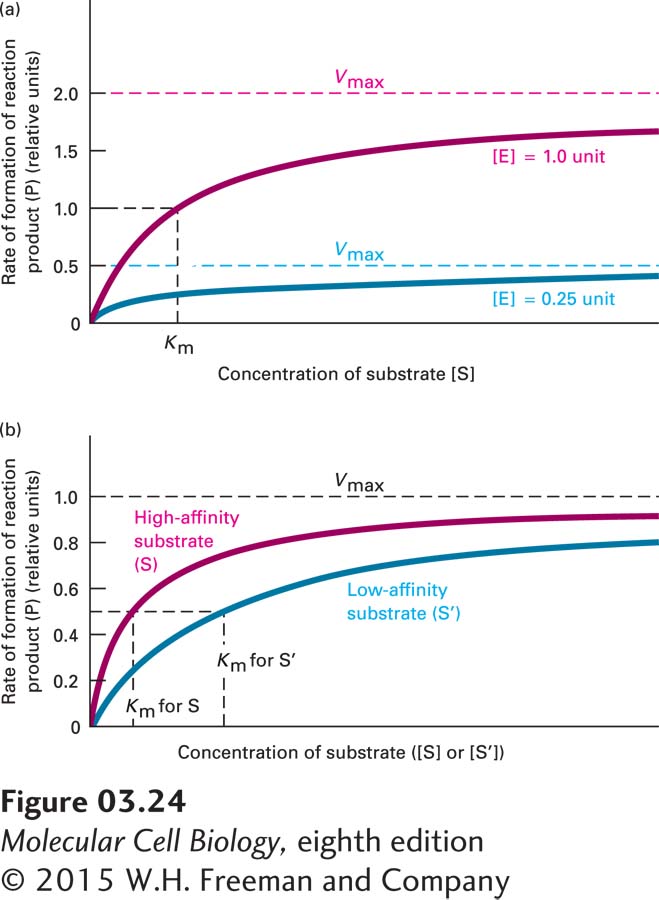
FIGURE 3- e- e- f- w-