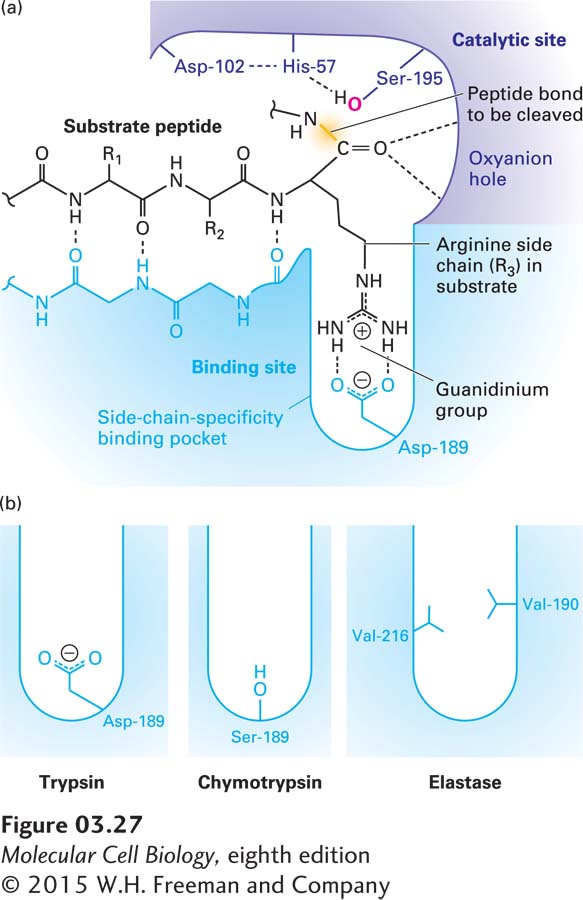
FIGURE 3- 27 Substrate binding in the active site of trypsin- like serine proteases. (a) The active site of trypsin (purple and blue molecule) with a bound substrate (black molecule). The substrate forms a two- stranded β sheet with trypsin’s substrate- binding site, and the side chain of an arginine (R3) in the substrate is bound in the side- chain- specificity binding pocket of the binding site. Its positively charged guanidinium group is stabilized by the negative charge on the side chain of the enzyme’s Asp- 189. This binding aligns the peptide bond of the arginine appropriately for hydrolysis catalyzed by the enzyme’s active- site catalytic triad (side chains of Ser- 195, His- 57, and Asp- 102). (b) The amino acids lining the side- chain- specificity binding pocket determine its shape and charge and thus its binding properties. Trypsin accommodates the positively charged side chains of arginine and lysine; chymotrypsin, large, hydrophobic side chains such as phenylalanine; and elastase, small side chains such as glycine and alanine. See J. J. Perona and C. S. Craik, 1997, J. Biol. Chem. 272:29987– 29990.
[Leave] [Close]