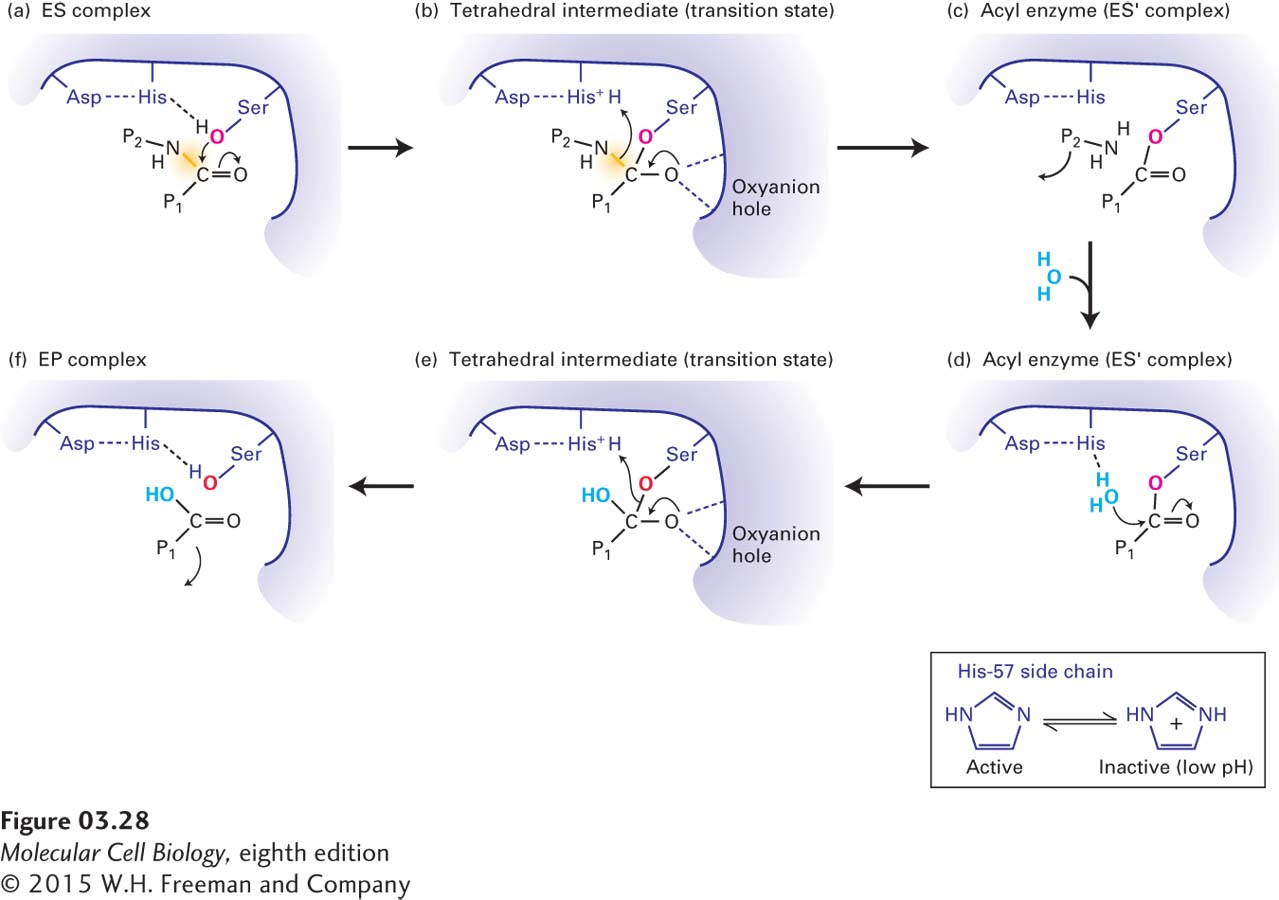
FIGURE 3- 28 Mechanism of serine protease– mediated hydrolysis of peptide bonds. The catalytic triad of Ser- 195, His- 57, and Asp- 102 in the active sites of serine proteases employs a multistep mechanism to hydrolyze peptide bonds in target proteins. (a) After a polypeptide substrate binds to the active site (see Figure 3- 27 ), forming an ES complex, the hydroxyl oxygen of Ser- 195 attacks the carbonyl carbon of the substrate’s targeted peptide bond (yellow). Movements of electrons are indicated by arrows. (b) This attack results in the formation of a transition state called the tetrahedral intermediate, in which the negative charge on the substrate’s oxygen is stabilized by hydrogen bonds formed with the enzyme’s oxyanion hole. (c) Additional electron movements result in the breaking of the peptide bond, release of one of the reaction products (NH2 — P2), and formation of the acyl enzyme (ES′ complex). (d) An oxygen from a solvent water molecule then attacks the carbonyl carbon of the acyl enzyme. (e) This attack results in the formation of a second tetrahedral intermediate. (f) Additional electron movements result in the breaking of the Ser- 195– substrate bond (formation of the EP complex) and release of the final reaction product (P1 — COOH). The side chain of His- 57, which is held in the proper orientation by hydrogen bonding to the side chain of Asp- 102, facilitates catalysis by withdrawing and donating protons throughout the reaction (inset). If the pH is too low and the side chain of His- 57 is protonated, it cannot participate in catalysis and the enzyme is inactive.
[Leave] [Close]