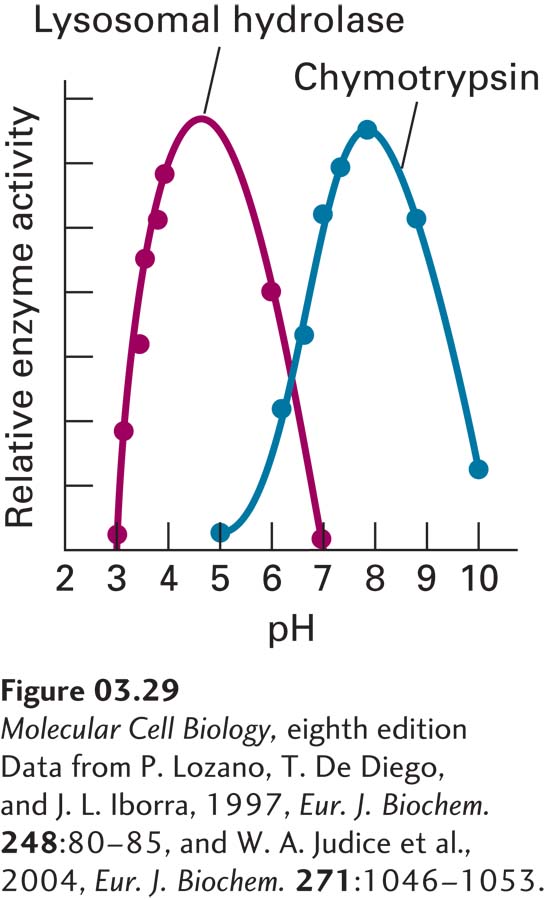
FIGURE 3- H- e- s-
[Data from P. Lozano, T. De Diego, and J. L. Iborra, 1997, Eur. J. Biochem. 248:80– 6–