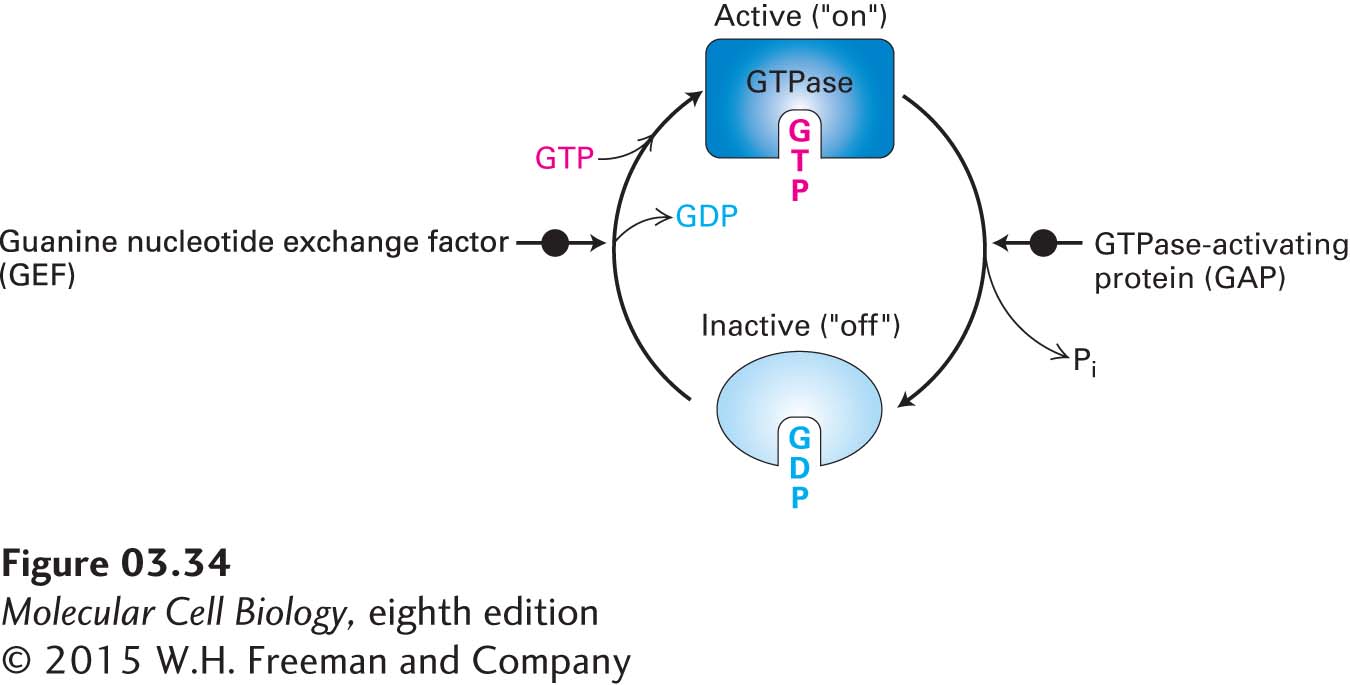
FIGURE 3- 34 The GTPase switch. GTPases are enzymes that bind to GTP and hydrolyze it to GDP. When bound to GTP, the GTPase protein adopts its active, or “on,” conformation and can interact with target proteins to regulate their activities. When the bound GTP is hydrolyzed to GDP by the intrinsic GTPase activity of the protein, the GTPase with GDP bound assumes an inactive, or “off,” conformation. The GTPase switch can be turned back on when another protein, called a GEF (guanine nucleotide exchange factor), mediates the replacement (exchange) of the bound GDP with a GTP molecule from the surrounding fluid. GTPase- activating proteins, or GAPs, can influence the rates of GTP hydrolysis. The binding of the active form of the GTPase to its targets is a form of noncovalent regulation.
[Leave] [Close]