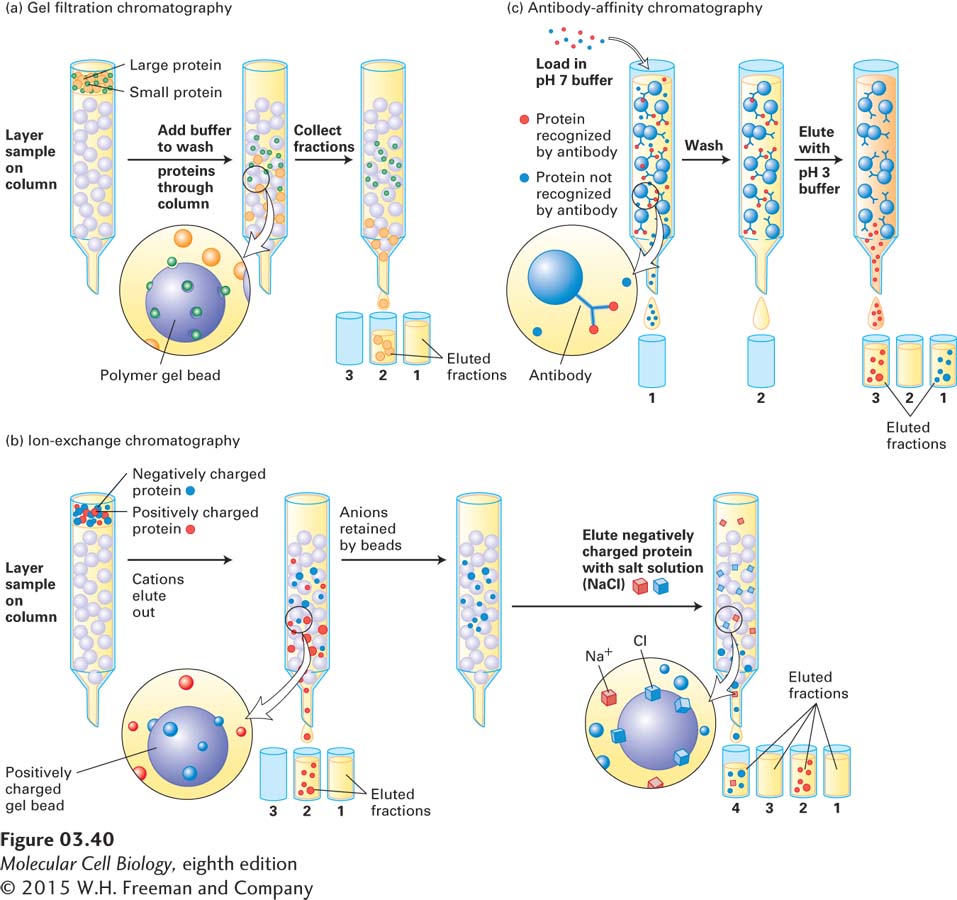
EXPERIMENTAL FIGURE 3- n- s— s— y- n-