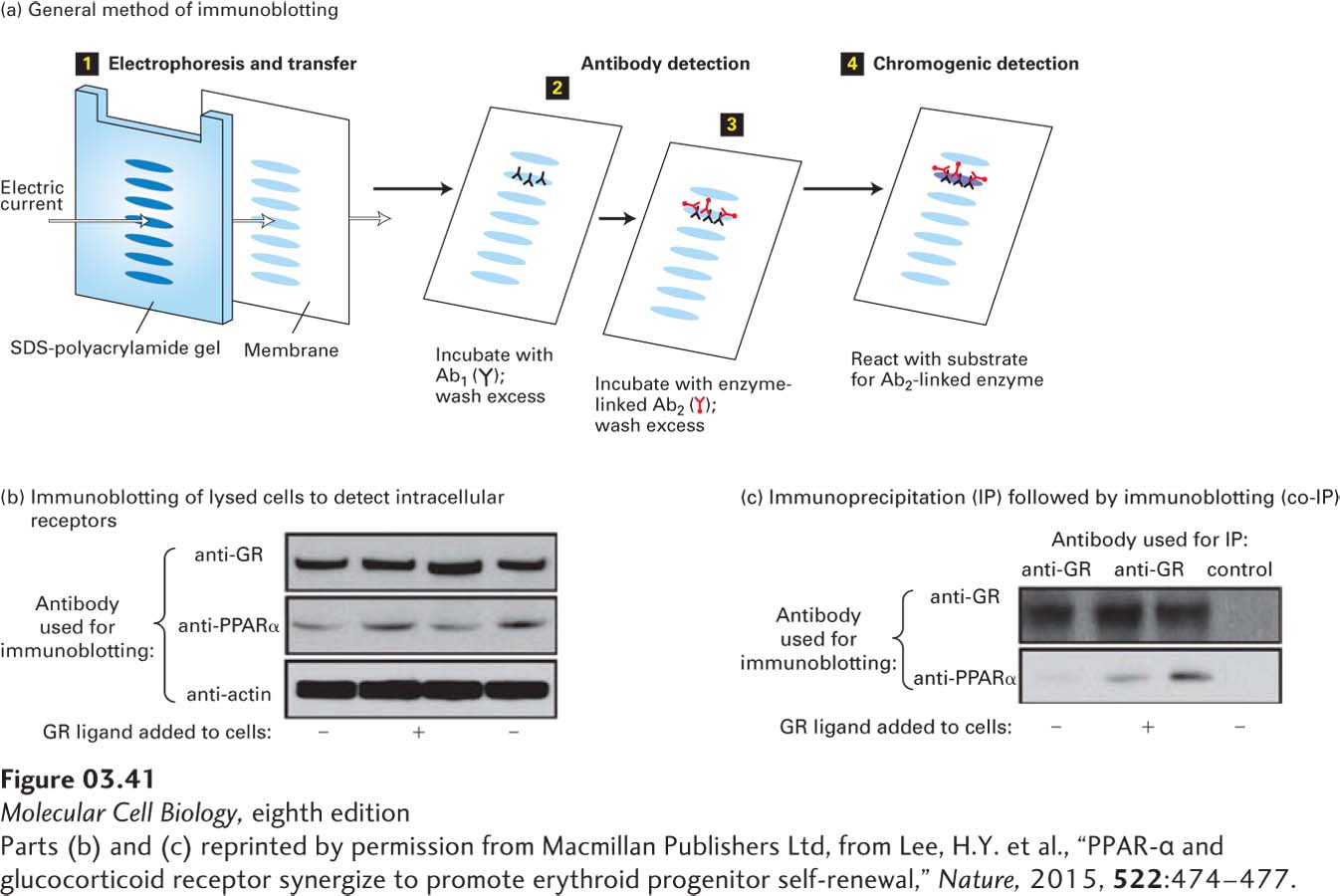
EXPERIMENTAL FIGURE 3- o- o- o- i- i- i- o- i- i- i- i- o-
[Parts (b) and (c) reprinted by permission from Macmillan Publishers Ltd, from Lee, H.Y. et al., “PPAR- f- 4–