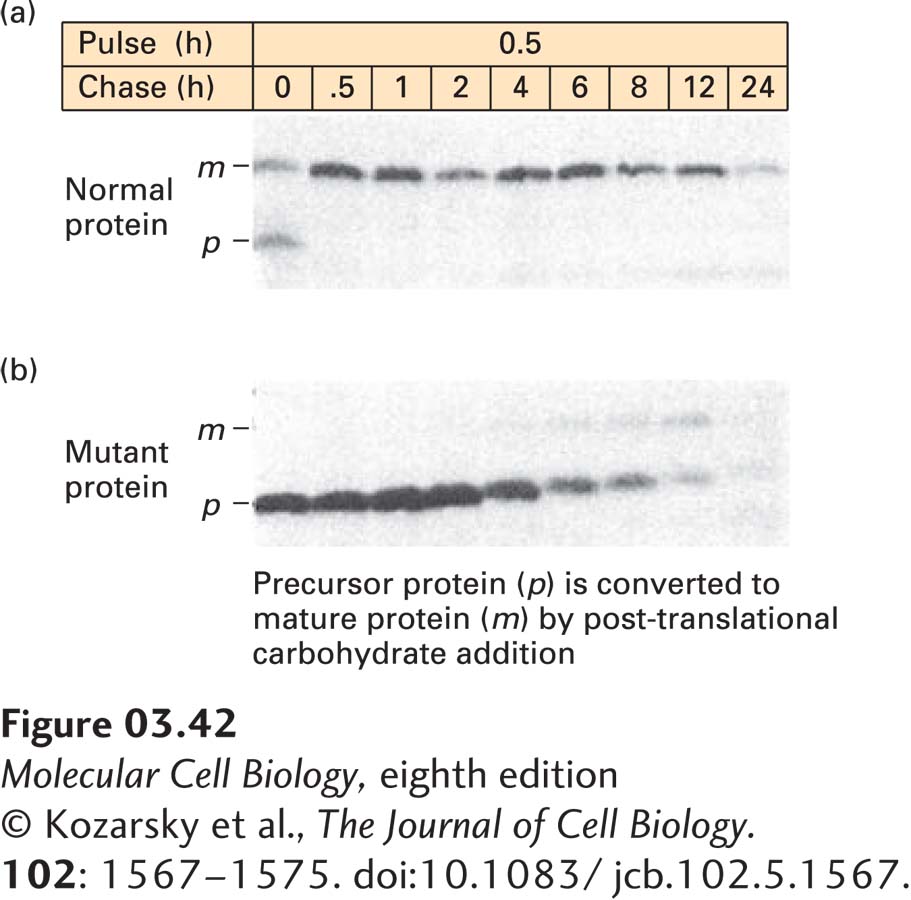
EXPERIMENTAL FIGURE 3- 42 Pulse- chase experiments can track the pathway of protein modification within cells. (a) To follow the fate of a specific newly synthesized protein in cells, cells were incubated with [35S]methionine for 0.5 hours (the pulse) to label all newly synthesized proteins, and any radioactive amino acid not incorporated into the cells was then washed away. The cells were further incubated (the chase) for varying times up to 24 hours, and samples from each time of chase were subjected to immunoprecipitation to isolate one specific protein (here the low- density lipoprotein receptor). SDS- PAGE of the immunoprecipitates followed by autoradiography permitted visualization of the target protein, which is initially synthesized as a small precursor (p) and then rapidly modified to a larger mature form (m) by addition of carbohydrates. About half of the labeled protein was converted from p to m during the pulse; the rest was converted after 0.5 hours of chase. The protein remained stable for 6– 8 hours before it began to be degraded (as indicated by reduced band intensity). (b) The same experiment was performed in cells in which a mutant form of the protein is made. The mutant p form cannot be properly converted to the m form, and it is more quickly degraded than the normal protein.
[© Kozarsky et al., The Journal of Cell Biology. 102: 1567– 1575. doi:10.1083/jcb.102.5.1567.]
[Leave] [Close]