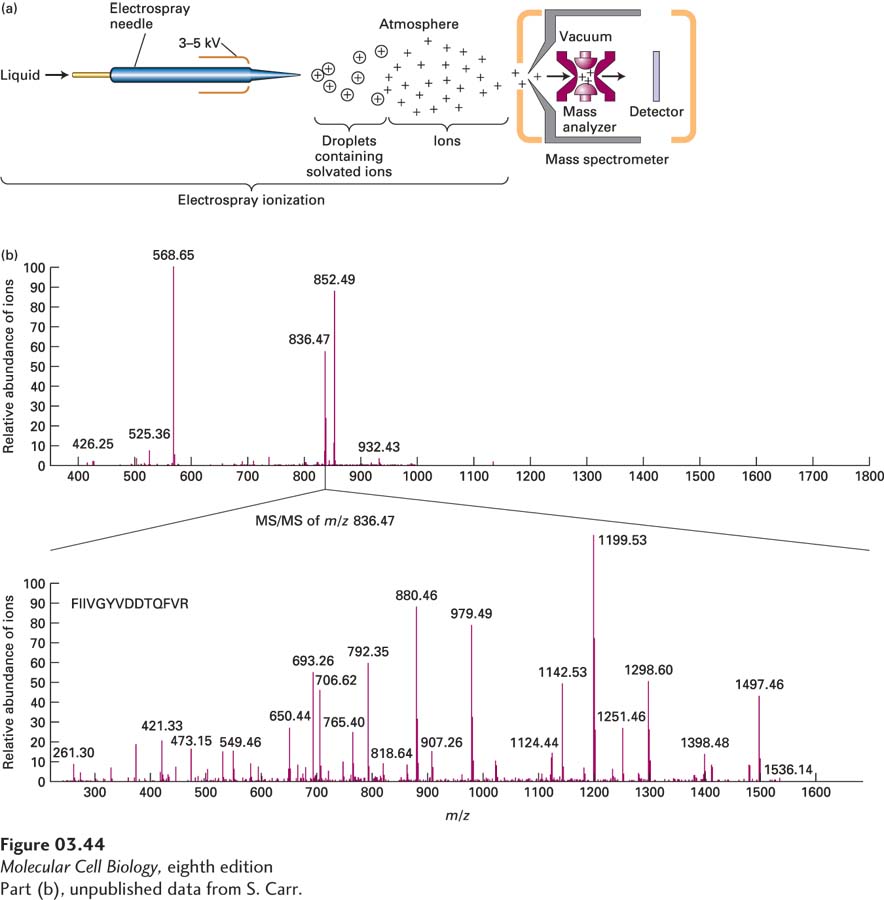
EXPERIMENTAL FIGURE 3- 44 Molecular mass of proteins and peptides can be determined by electrospray ionization ion- trap mass spectrometry. (a) Electrospray (ES) ionization converts proteins and peptides in a solution into highly charged gaseous ions by passing the solution through a needle (forming the droplets) that has a high voltage across it (charging the droplets). Evaporation of the solvent produces gaseous ions that enter a mass spectrometer. The ions are analyzed by an ion- trap mass analyzer that then directs ions to the detector. (b) Top panel: Mass spectrum of a mixture of three major and several minor peptides from the mouse H- 2 class I histocompatibility antigen Q10 α chain is presented as the relative abundance of the ions striking the detector (y axis) as a function of the mass- to- charge (m/z) ratio (x axis). Bottom panel: In an MS/MS instrument such as the ion trap shown in part (a), a specific peptide ion can be selected for fragmentation into smaller ions that are then analyzed and detected. The MS/MS spectrum (also called the product- ion spectrum) provides detailed structural information about the parent ion, including sequence information for peptides. Here the ion with an m/z of 836.47 was selected and fragmented and the m/z mass spectrum of the product ions measured. Note there is no longer an ion with an m/z of 836.47 present because it was fragmented. From the varying sizes of the product ions, the understanding that peptide bonds are often broken in such experiments, the known m/z values for individual amino acid fragments, and database information, the sequence of the peptide, FIIVGYVDDTQFVR, can be deduced.
[Part (b), unpublished data from S. Carr.]
[Leave] [Close]