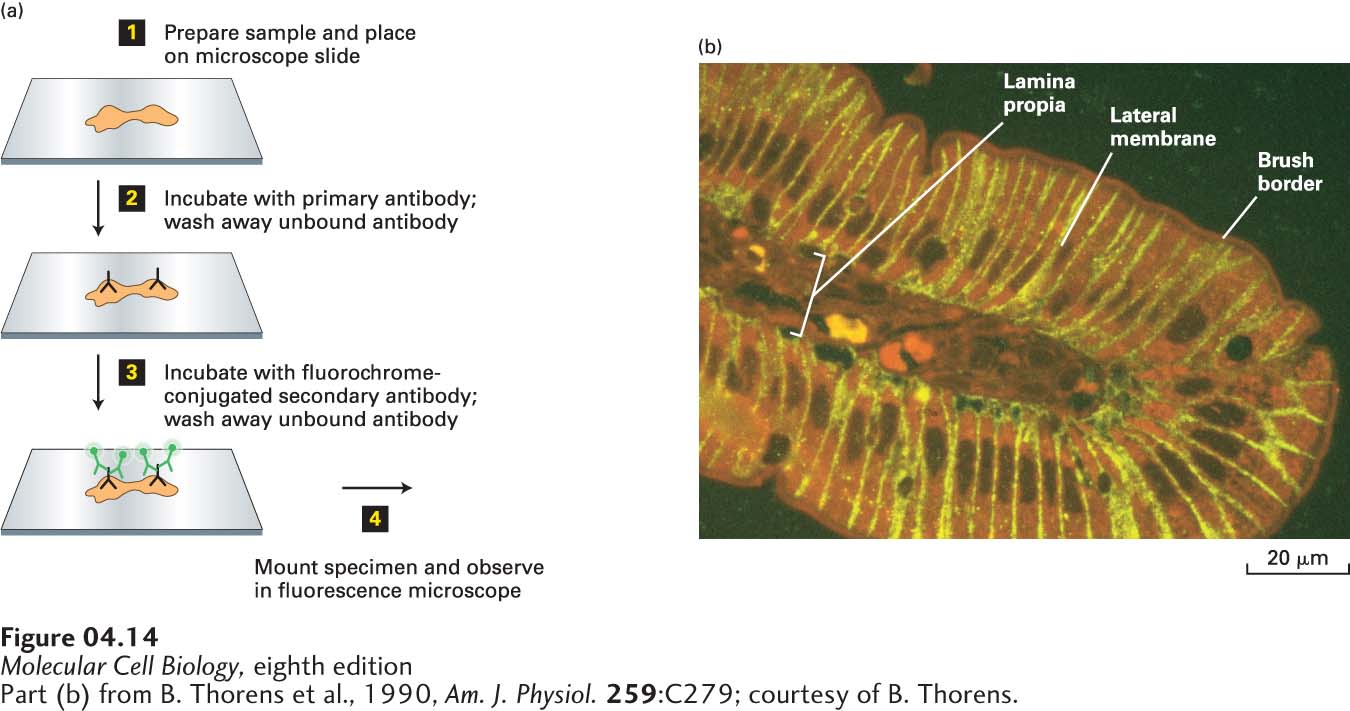
FIGURE 4- 14 A specific protein can be localized in fixed tissue sections by indirect immunofluorescence microscopy. (a) To localize a protein by immunofluorescence microscopy, a tissue section, or sample of cells, must be chemically fixed and made permeable to antibodies (step 1). The sample is then incubated with a primary antibody that binds specifically to the antigen of interest, and unbound antibody is then removed by washing (step 2). The sample is next incubated with a fluorochrome- labeled secondary antibody that specifically binds to the primary antibody, and again, excess secondary antibody is removed by washing (step 3). The sample is then mounted in specialized mounting medium and examined in a fluorescence microscope (step 4). (b) In this example, a section of the rat intestinal wall was stained with Evans blue, which generates a nonspecific red fluorescence, and GLUT2, a glucose transport protein, was localized by indirect immunofluorescence microscopy. GLUT2 (yellow) is seen to be present in the basal and lateral sides of the intestinal cells, but is absent from the brush border, composed of closely packed microvilli on the apical surface facing the intestinal lumen. Capillaries run through the lamina propria, a loose connective tissue beneath the epithelial layer.
[Part (b) from B. Thorens et al., 1990, Am. J. Physiol. 259:C279; courtesy of B. Thorens.]
[Leave] [Close]