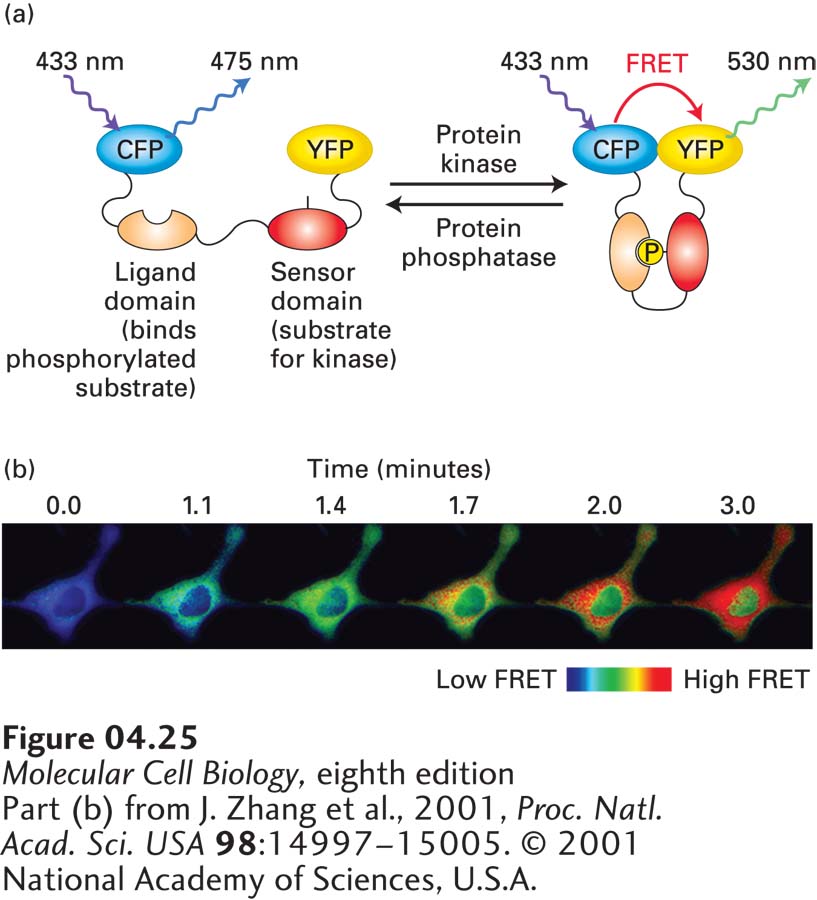
EXPERIMENTAL FIGURE 4- 25 FRET biosensors can detect local biochemical environments. (a) A FRET biosensor is a fusion protein containing two fluorescent proteins linked by a region sensitive to the environment under study. In this example, a protein construct consists of CFP linked to YFP by a region that contains a particular sequence that can be phosphorylated by a specific kinase (the “sensor domain”) and a region (the “ligand domain”) that binds the sensor domain when it is phosphorylated. In the absence of kinase activity, the two fluorescent proteins are too far apart to undergo FRET, whereas when locally phosphorylated by the active kinase, the sensor domain becomes phosphorylated, the ligand domain binds to it, and CFP and YFP are brought sufficiently close to undergo FRET. The sensor can also be deactivated when it encounters the appropriate phosphatase that removes the added phosphate. Thus the biosensor reports on the ratio of kinase to phosphatase activity in the local environment. (b) An example of the use of a FRET biosensor for protein kinase A, which is activated by elevation of cAMP. In this example, forskolin, a drug that induces the generation of cAMP, was added to cells at t = 0 and the images collected at various times thereafter. Imaging shows both the rate of activation and localization of the active kinase.
[Part (b) from J. Zhang et al., 2001, Proc. Natl. Acad. Sci. USA 98:14997– 15005. © 2001 National Academy of Sciences, U.S.A.]
[Leave] [Close]