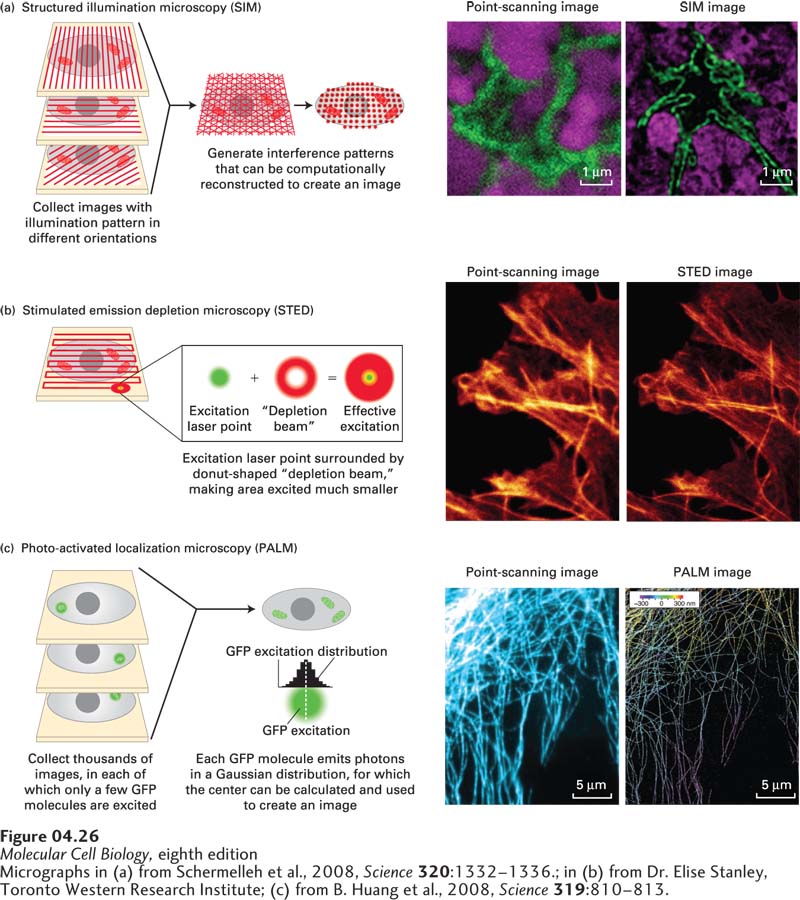
EXPERIMENTAL FIGURE 4- 26 Super- resolution microscopy can generate light- microscope images with up to nanometer resolution. The theoretical resolution of the light microscope can be circumvented by super- resolution microscopy. (a) In structured illumination microscopy (SIM), the sample is illuminated by a pattern of light and dark stripes and several images are taken as the illumination is rotated. This technique generates interference patterns that can be mathematically reconstructed to generate a higher- resolution image. The images on the right show the similar fields of the nucleus (lamin, green; DNA, magenta) imaged by conventional point- scanning confocal microscopy and by SIM, which improves resolution about twofold. (b) In stimulated emission depletion microscopy (STED), the sample is scanned as in point- scanning microscopy, but with a very small point of light, generated by an emission laser and confined by a donut- shaped stimulated emission depletion zone. The sample at the right shows part of a cell stained for actin fibers after imaging by point- scanning microscopy and by STED. (c) In photoactivated localization microscopy (PALM), use is made of a variant of GFP that can be photoactivated by a wavelength different from its excitation wavelength. When a small number of GFP molecules are activated, and then excited, each will emit thousands of photons that can be collected. This generates a Gaussian curve centered on the location of the emitting GFP; the center provides the location of the GFP to nanometer accuracy. This process is reiterated hundreds of times to excite other GFP molecules, and a high- resolution image emerges. At the right, a confocal image of microtubules is compared with a corresponding super- resolution image in which the three- dimensional arrangement of the microtubules is color coded.
[Micrographs in (a) from Schermelleh et al., 2008, Science 320:1332– 1336.; in (b) from Dr. Elise Stanley, Toronto Western Research Institute; (c) from B. Huang et al., 2008, Science 319:810– 813.]
[Leave] [Close]