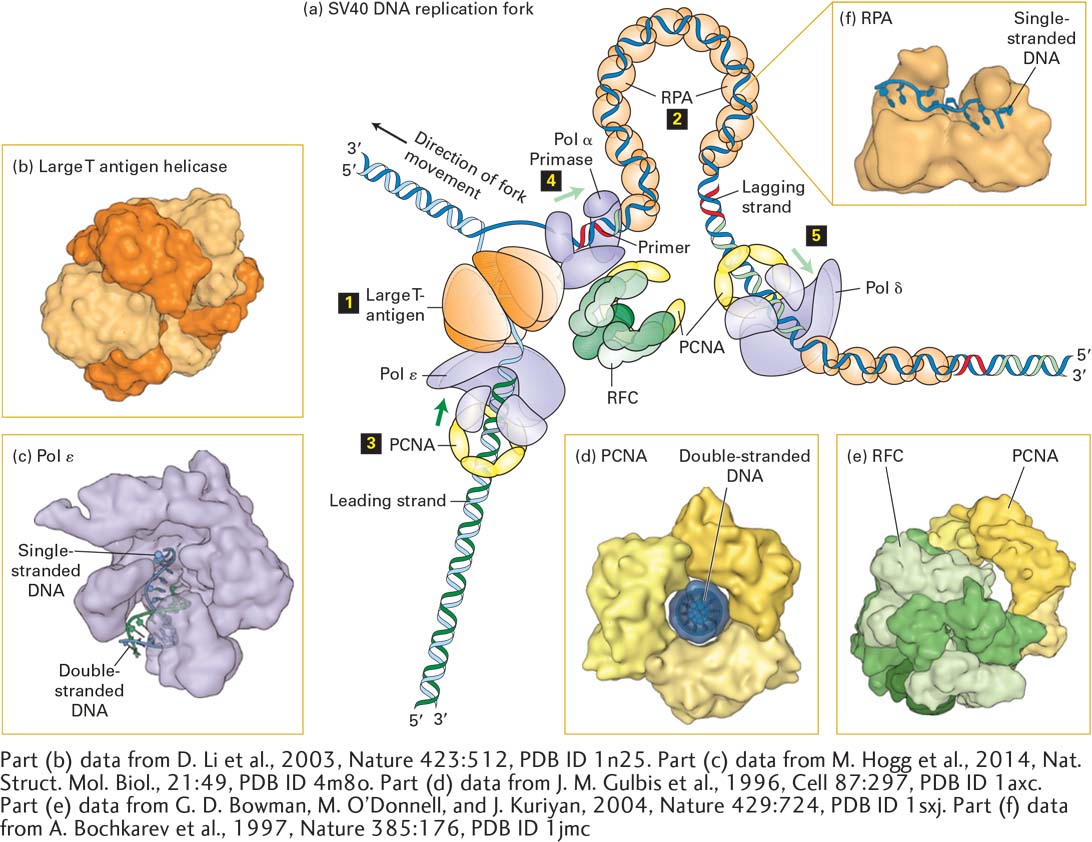
FIGURE 5- 30 Model of an SV40 DNA replication fork. (a) A hexamer of large T- antigen, a viral protein, functions as a helicase to unwind the parent DNA strands. The leading strand is extended by DNA polymerase ε (Pol ε) up to the replication fork. Pol ε is bound to a ring of PCNA that surrounds the daughter double- stranded DNA so that the Pol ε-PCNA complex remains stably associated with the replication fork. The single- stranded region of the lagging strand template generated by T- antigen helicase is bound by multiple copies of the heterotrimeric protein RPA. Primers for lagging- strand synthesis (red, RNA; light green, DNA) are synthesized by a complex of primase and DNA polymerase α (Pol α). The 3′ end of each primer synthesized by Pol α–primase is then bound by a PCNA– Pol δ complex, which extends the primer and synthesizes most of each Okazaki fragment. (b) The helicase domain of SV40 T- antigen forms a hexameric replicative helicase. Subunits are shown in alternating light and dark orange. (c) Model of DNA polymerase ε extending the 3’-end of the leading strand. (d) The three subunits of PCNA, shown in different shades of yellow, form a circular structure with a central hole through which daughter double- stranded DNA passes. (e) RFC, the pentameric “clamp- loader” (monomers shown in different shades of green) is shown bound to a circular trimer PCNA before the PCNA “clamp” is opened. (f) The large subunit of RPA contains two domains that bind single- stranded DNA. Note that the single DNA strand is extended, with the bases oriented in an optimal conformation for replication by Pol δ. See M. O’Donnell, L. Langston, and B. Stillman, 2013, Cold Spring Harbor Perspect. Biol. 5:a010108.
[Part (b) data from D. Li et al., 2003, Nature 423:512, PDB ID 1n25. Part (c) data from M. Hogg et al., 2014, Nat. Struct. Mol. Biol., 21:49, PDB ID 4m8o. Part (d) data from J. M. Gulbis et al., 1996, Cell 87:297, PDB ID 1axc. Part (e) data from G. D. Bowman, M. O’Donnell, and J. Kuriyan, 2004, Nature 429:724, PDB ID 1sxj. Part (f) data from A. Bochkarev et al., 1997, Nature 385:176, PDB ID 1jmc.]
[Leave] [Close]