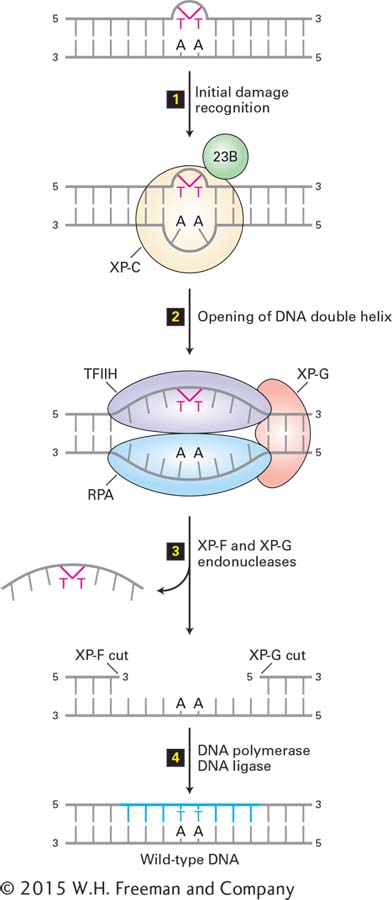
FIGURE 5- 38 Nucleotide excision repair in human cells. A DNA lesion that causes distortion of the double helix, such as a thymine- thymine dimer, is initially recognized by a complex of the XP- C (xeroderma pigmentosum C protein) and 23B proteins (step 1). This complex then recruits transcription factor TFIIH, whose helicase subunits, powered by ATP hydrolysis, partially unwind the double helix. XP- G and RPA proteins then bind to the complex and further unwind and stabilize the helix until a bubble of about 25 bases is formed (step 2). Then XP- G (now acting as an endonuclease) and XP- F, a second endonuclease, cut the damaged strand at points 24– 32 bases apart on each side of the lesion (step 3). This releases the DNA fragment with the damaged bases, which is degraded to mononucleotides. Finally the gap is filled by DNA polymerase exactly as in DNA replication, and the remaining nick is sealed by DNA ligase (step 4). See J. Hoeijmakers, 2001, Nature 411:366, and O. Schärer, 2003, Angewandte Chemie 42:2946.
[Leave] [Close]