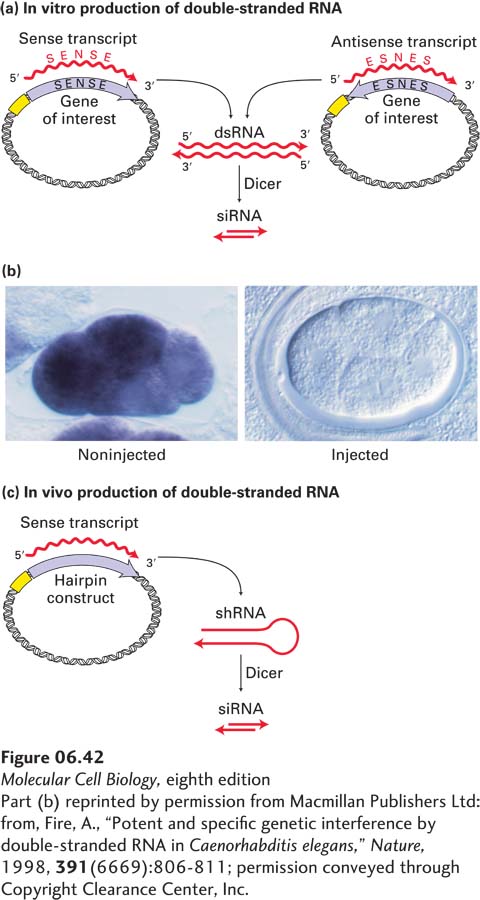
EXPERIMENTAL FIGURE 6- 42 RNA interference (RNAi) can inhibit gene function in C. elegans and other organisms. (a) In vitro production of double- stranded RNA (dsRNA) for interference with a specific target gene. The coding sequence of the gene, derived from either a cDNA clone or a segment of genomic DNA, is placed in two orientations in a plasmid vector adjacent to a strong promoter. Transcription of both constructs in vitro using RNA polymerase and ribonucleoside triphosphates yields many RNA copies in both the sense orientation (identical to the mRNA sequence) and the complementary antisense orientation. Under suitable conditions, these complementary RNA molecules hybridize to form dsRNA. When the dsRNA is injected into cells, it is cleaved by Dicer into siRNAs. (b) Inhibition of mex3 RNA expression in C. elegans embryos by RNAi (see the text for the mechanism). Expression of mex3 RNA in embryos was assayed by in situ hybridization to a probe specific for this mRNA, linked to an enzyme that produces a colored (purple) product. (Left) Wild- type embryo. (Right) Embryo derived from a worm injected with double- stranded mex3 RNA. Each four- cell- stage embryo is ∼50 mm in length. (c) In vivo production of double- stranded RNA via an engineered plasmid introduced directly into cells. The synthetic gene construct is a tandem arrangement of both sense and antisense sequences of the target gene. When it is transcribed, double- stranded small hairpin RNA (shRNA) forms. The shRNA is cleaved by Dicer to form siRNA.
[Part (b) reprinted by permission from Macmillan Publishers Ltd: from, Fire, A., “Potent and specific genetic interference by double- stranded RNA in Caenorhabditis elegans,” Nature, 1998, 391(6669):806- 811; permission conveyed through Copyright Clearance Center, Inc.]
[Leave] [Close]