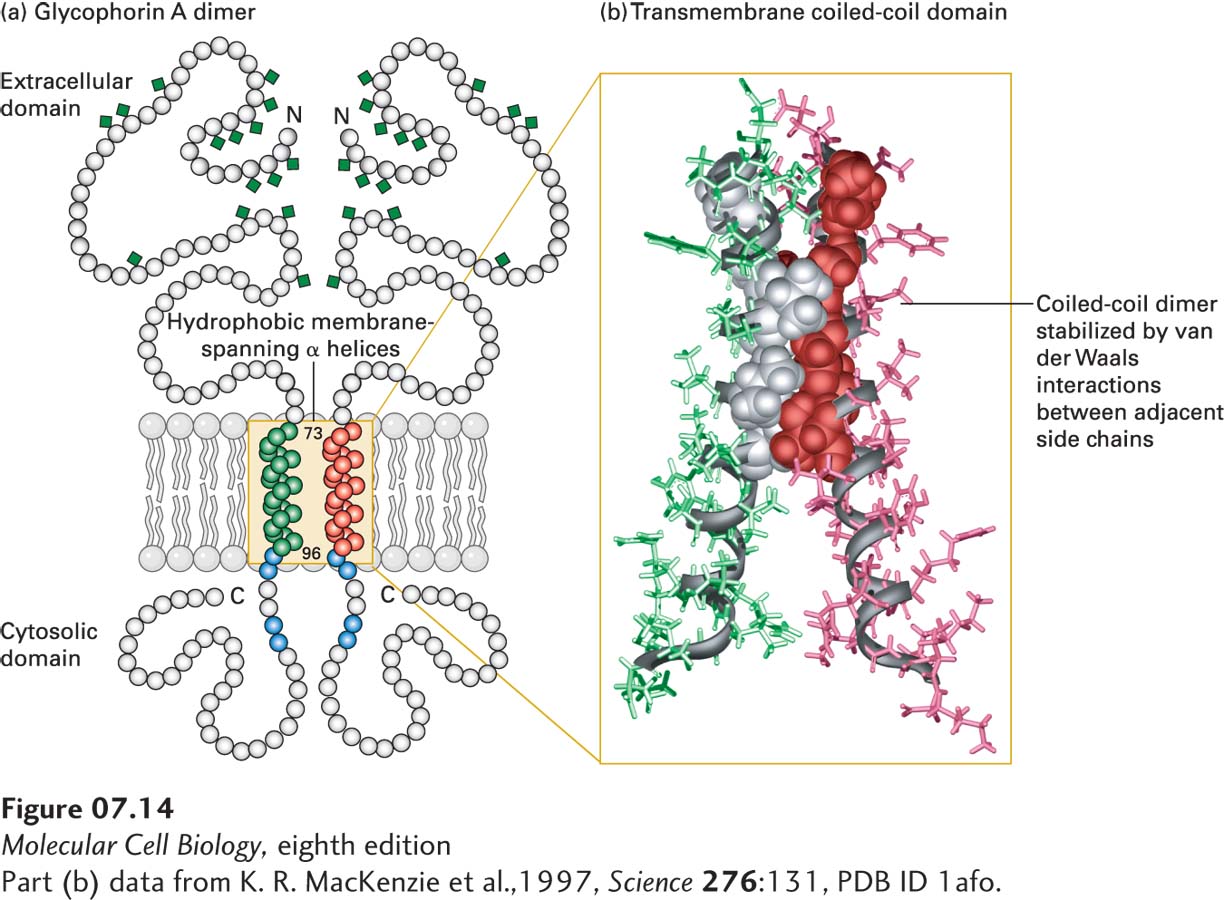
FIGURE 7- e- 3- e- 3– e- d-
[Part (b) data from K. R. MacKenzie et al., 1997, Science 276:131, PDB ID 1afo.]