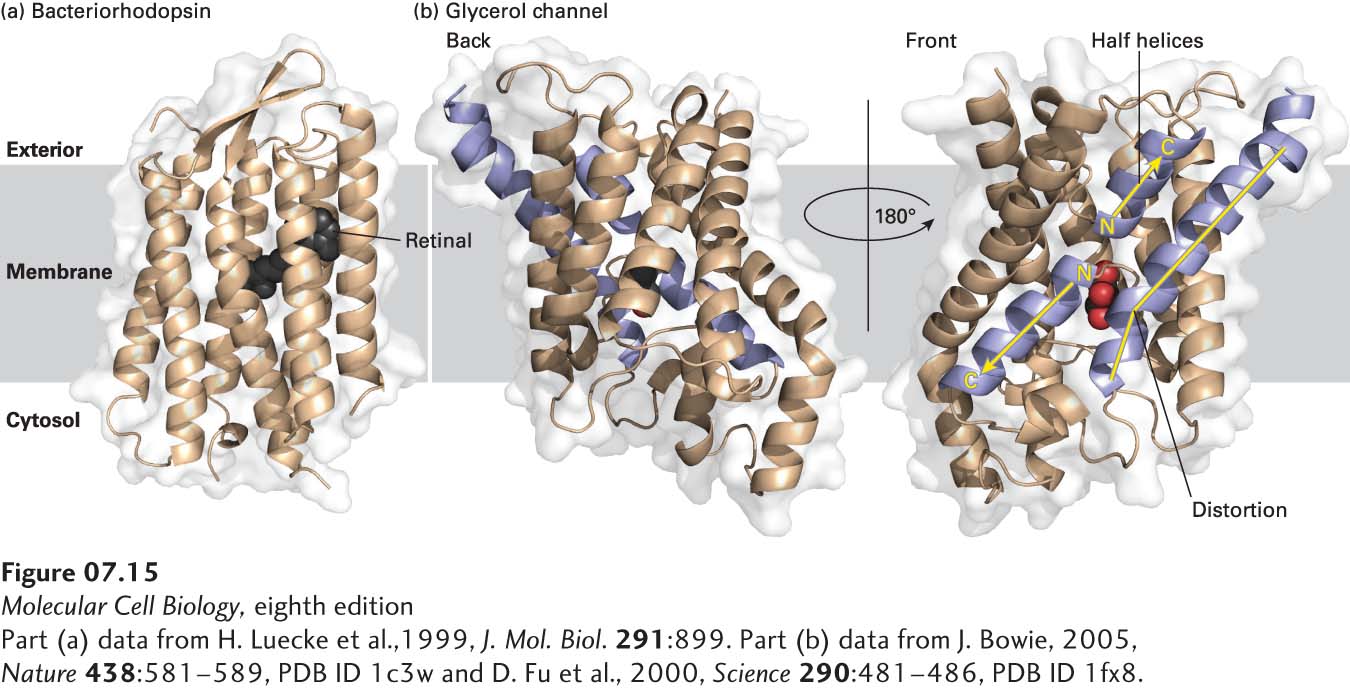
FIGURE 7- n– e- e- e- e- 3-
[Part (a) data from H. Luecke et al., 1999, J. Mol. Biol. 291:899. Part (b) data from J. Bowie, 2005, Nature 438:581– 1–