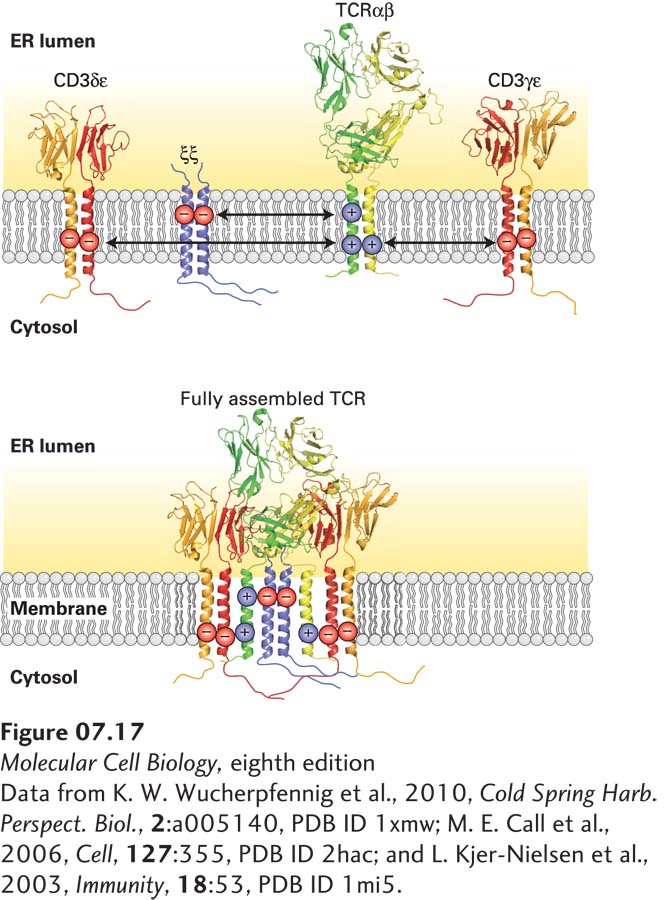
FIGURE 7- e- r-
[Data from K. W. Wucherpfennig et al., 2010, Cold Spring Harb. Perspect. Biol., 2:a005140, PDB ID 1xmw; M. E. Call et al., 2006, Cell, 127:355, PDB ID 2hac; and L. Kjer-