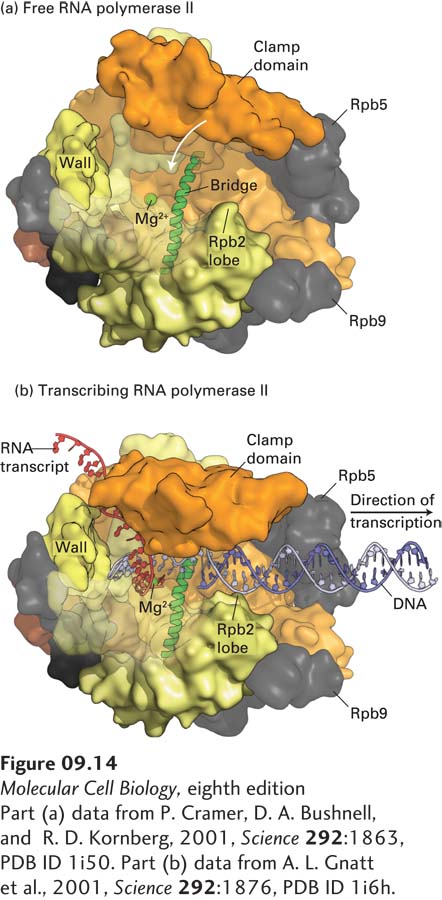
FIGURE 9- 14 The clamp domain of RPBI. The structures of the free (a) and transcribing (b) RNA polymerase II differ mainly in the position of a clamp domain in the RPB1 subunit (orange), which swings over the cleft between the jaws of the polymerase during formation of the transcribing complex, trapping the template DNA strand and transcript. Binding of the clamp domain to the 8– 9- bp RNA- DNA hybrid may help couple clamp closure to the presence of RNA, stabilizing the closed, elongating complex. RNA is shown in red, and the template strand in light purple. For clarity, downstream nontemplate DNA is not shown. The clamp closes over the incoming downstream DNA. Portions of RBP2 that form one side of the cleft have been removed so that the nucleic acids can be better visualized. The Mg2+ ion that participates in catalysis of phosphodiester bond formation is shown in green. Wall is the domain of RPB2 that forces the template DNA entering the jaws of the polymerase to bend before it exits the polymerase. The bridge α helix, shown in green, extends across the cleft in the polymerase (see Figure 9- 12b ) and is postulated to bend and straighten as the polymerase translocates one base down the template strand. The nontemplate strand is thought to form a flexible single- stranded region above the cleft (not shown), extending from three bases downstream of the template base- paired to the 3′ base of the growing RNA to where the template strand exits the polymerase, where it hybridizes with the template strand to generate the transcription bubble.
[Part (a) data from P. Cramer, D. A. Bushnell, and R. D. Kornberg, 2001, Science 292:1863, PDB ID 1i50. Part (b) data from A. L. Gnatt et al., 2001, Science 292:1876, PDB ID 1i6h.]
[Leave] [Close]