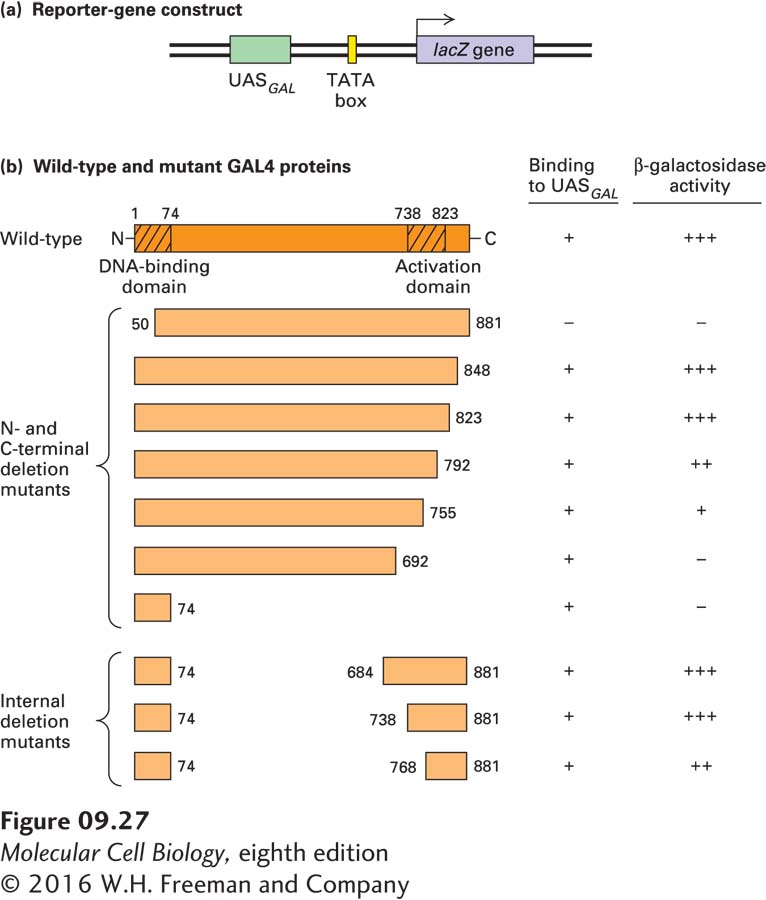
EXPERIMENTAL FIGURE 9- r- 4- r- d- d- d- N- C- A- N- C- C-