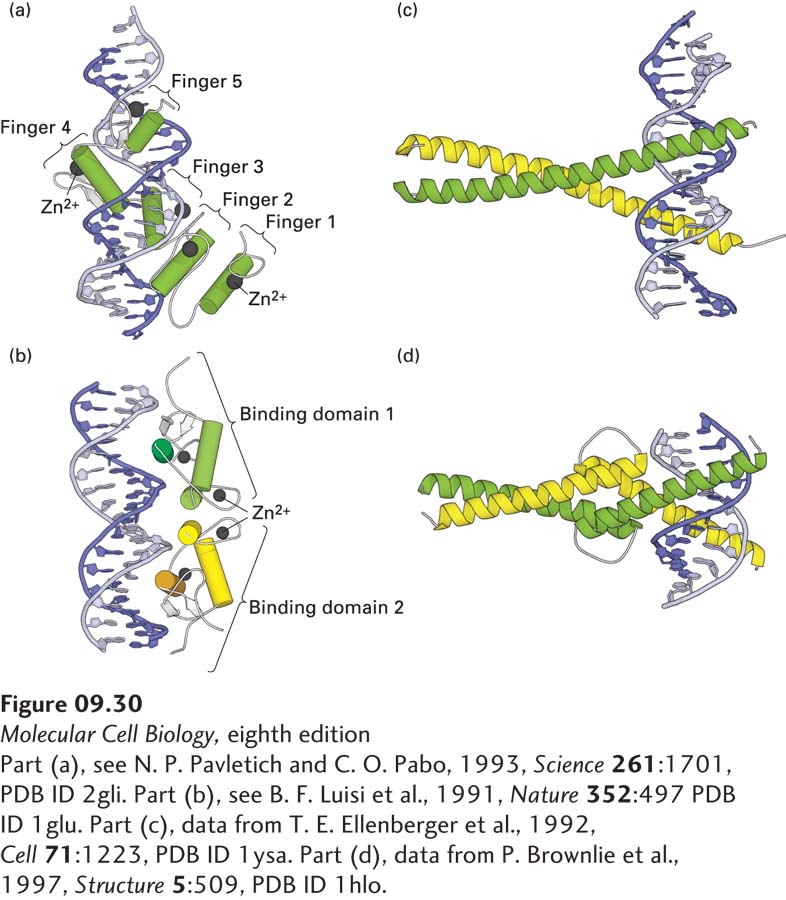
FIGURE 9- A- A- c- c- e- d- A- N- r– d-
[Part (a), see N. P. Pavletich and C. O. Pabo, 1993, Science 261:1701, PDB ID 2gli. Part (b), see B. F. Luisi et al., 1991, Nature 352:497 PDB ID 1glu. Part (c), data from T. E. Ellenberger et al., 1992, Cell 71:1223, PDB ID 1ysa. Part (d), data from P. Brownlie et al., 1997, Structure 5:509, PDB ID 1hlo.]