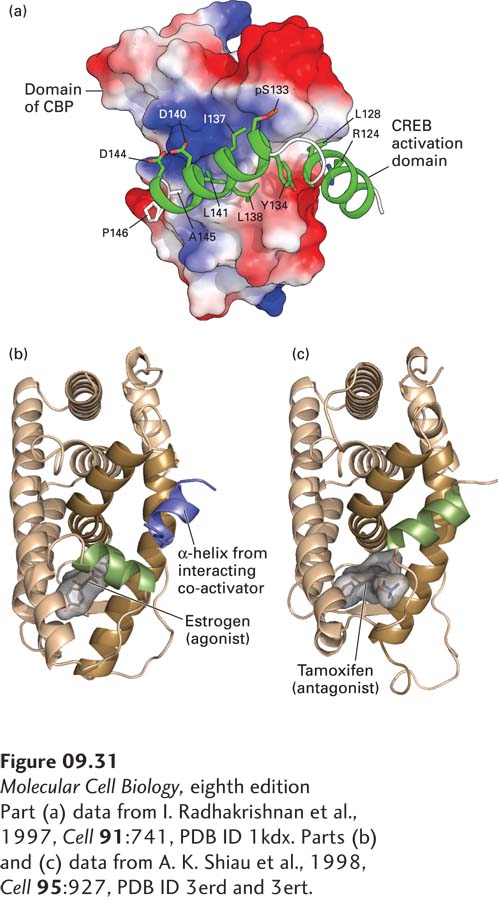
FIGURE 9- o- t- o- e- d- d- d- o- o- r– o-
[Part (a) data from I. Radhakrishnan et al., 1997, Cell 91:741, PDB ID 1kdx. Parts (b) and (c) data from A. K. Shiau et al., 1998, Cell 95:927, PDB ID 3erd and 3ert.]