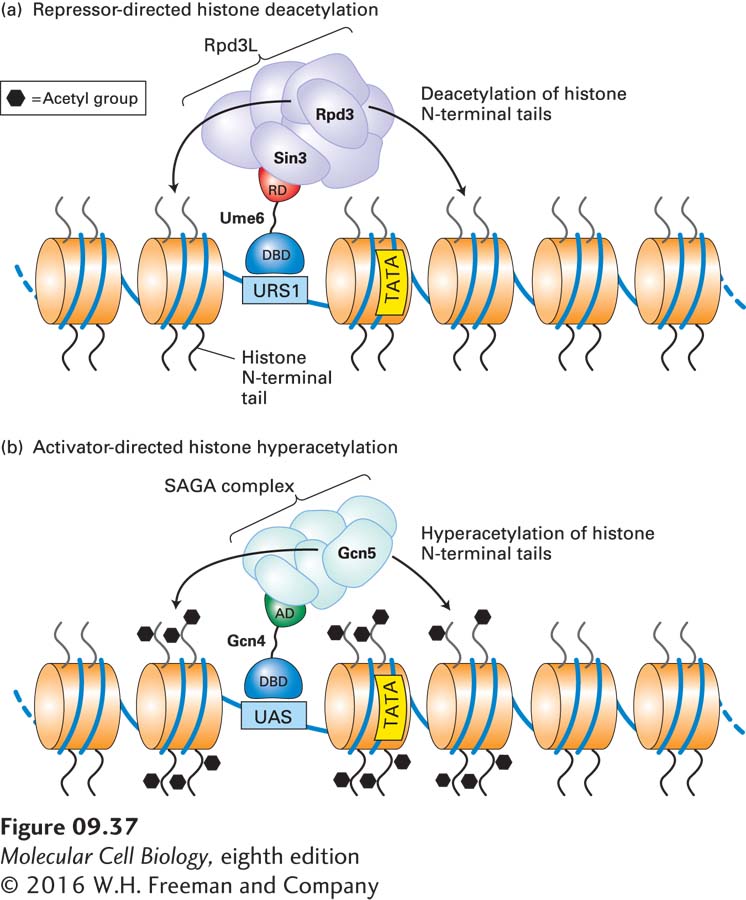
FIGURE 9- r- N- A- N- 6- r- N- A- N- 4-