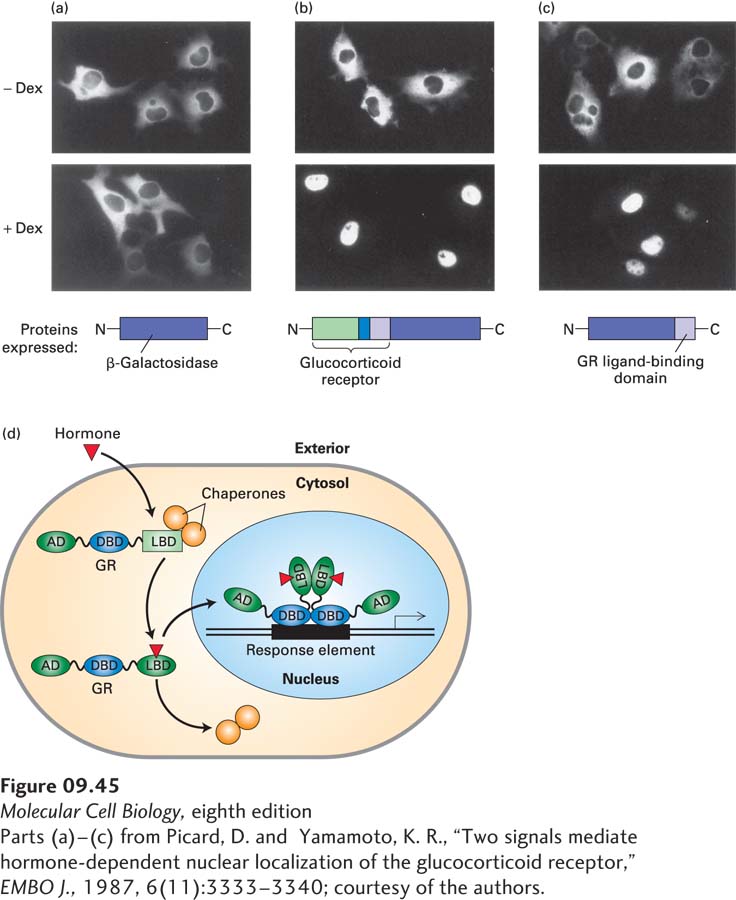
EXPERIMENTAL FIGURE 9- e- d- e- e- d- d- A- d- N-
[Parts (a)–(c) from Picard, D. and Yamamoto, K. R., “Two signals mediate hormone- 3–