Osmotic Pressure Causes Water to Move Across Membranes
Movement of water into and out of cells is an important feature of the life of all organisms. The aquaporins are a family of membrane proteins that allow water and a few other small uncharged molecules, such as glycerol, to cross cellular membranes efficiently. But before discussing these transport proteins, we need to review osmosis, the force that powers the movement of water across membranes.
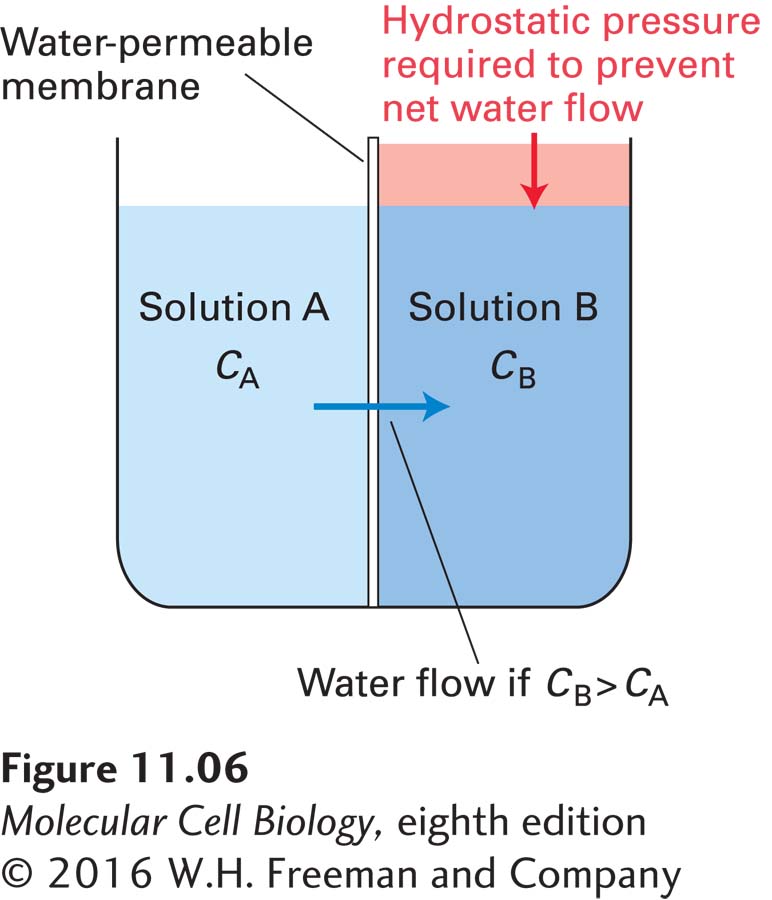
Water spontaneously moves “downhill” across a semipermeable membrane from a solution of lower solute concentration (relatively high water concentration) to one of higher solute concentration (relatively low water concentration), a process termed osmosis, or osmotic flow. In effect, osmosis is equivalent to “diffusion” of water across a semipermeable membrane. Osmotic pressure is defined as the hydrostatic pressure required to stop the net flow of water across a membrane separating solutions of different water concentrations (Figure 11-6). In other words, osmotic pressure balances the entropy-
The movement of water across the plasma membrane determines the volume of an individual cell, which must be regulated to avoid damage to the cell. Small changes in extracellular osmotic conditions cause most animal cells to swell or shrink rapidly. When placed in a hypotonic solution (i.e., one in which the concentration of non-
![]() In vascular plants, water and minerals are absorbed from the soil by the roots and move up the plant through conducting tubes (the xylem); water loss from the plant, mainly by evaporation from the leaves, drives this movement of water. Unlike animal cells, plant, algal, fungal, and bacterial cells are surrounded by a rigid cell wall, which resists the expansion of the volume of the cell when the intracellular osmotic pressure increases. Without such a wall, animal cells expand when internal osmotic pressure increases; if that pressure rises too much, the cells burst like overinflated balloons. Because of the cell wall, the osmotic influx of water that occurs when plant cells are placed in a hypotonic solution (even pure water) leads to an increase in intracellular pressure, but not in cell volume.
In vascular plants, water and minerals are absorbed from the soil by the roots and move up the plant through conducting tubes (the xylem); water loss from the plant, mainly by evaporation from the leaves, drives this movement of water. Unlike animal cells, plant, algal, fungal, and bacterial cells are surrounded by a rigid cell wall, which resists the expansion of the volume of the cell when the intracellular osmotic pressure increases. Without such a wall, animal cells expand when internal osmotic pressure increases; if that pressure rises too much, the cells burst like overinflated balloons. Because of the cell wall, the osmotic influx of water that occurs when plant cells are placed in a hypotonic solution (even pure water) leads to an increase in intracellular pressure, but not in cell volume.
In plant cells, the concentration of solutes (e.g., sugars and salts) is usually higher in the vacuole (see Figure 1-12a) than in the cytosol, which in turn has a higher solute concentration than the extracellular space. The osmotic pressure generated by the entry of water into the cytosol and then into the vacuole, called turgor pressure, pushes the cytosol and the plasma membrane against the resistant cell wall. Plant cells can harness this pressure to help them stand upright and grow. Cell elongation during growth occurs by means of a hormone-
Although most protozoans (like animal cells) do not have a rigid cell wall, many contain a contractile vacuole that permits them to avoid osmotic lysis. A contractile vacuole takes up water from the cytosol and, unlike a plant vacuole, periodically discharges its contents through fusion with the plasma membrane. Thus even though water continuously enters the protozoan cell by osmotic flow, the contractile vacuole prevents too much water from accumulating in the cell and swelling it to the bursting point.