Selective Movement of Ions Creates a Transmembrane Electric Gradient
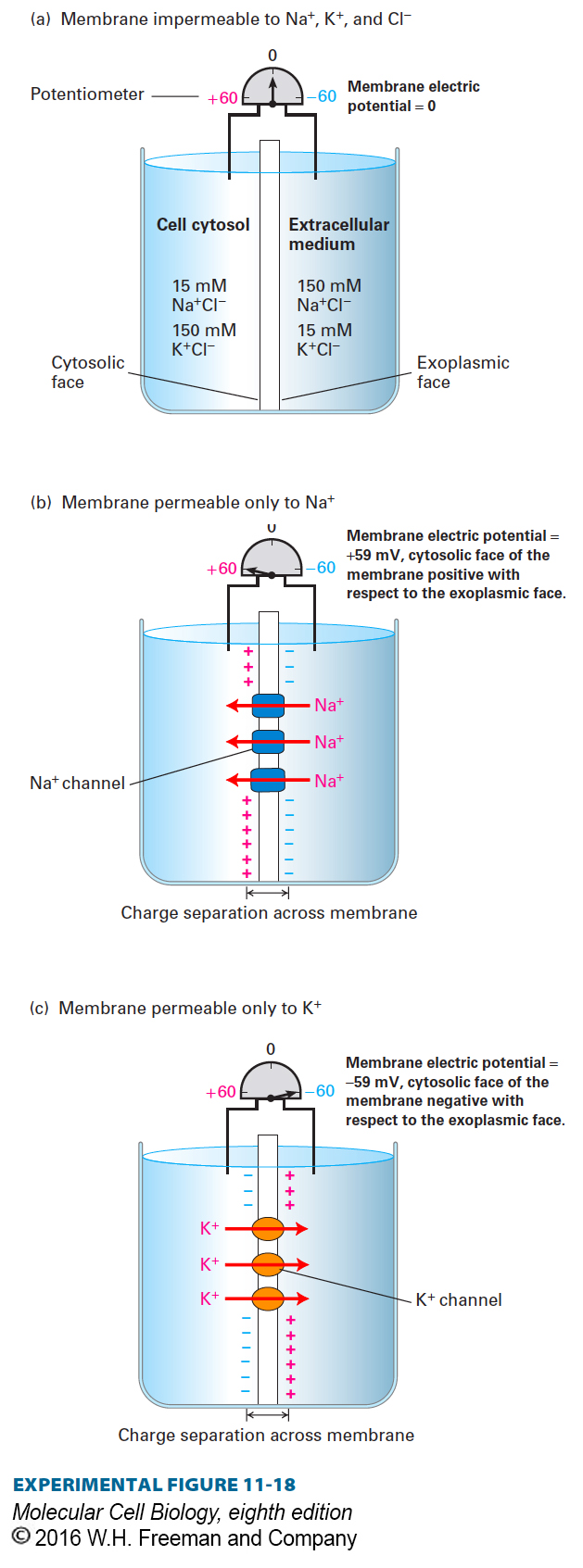
To help explain how an electric potential across the plasma membrane can arise, we first consider a set of simplified experimental systems in which a membrane separates a 150 mM NaCl/15 mM KCl solution (similar to the extracellular medium surrounding metazoan cells) on the right from a 15 mM NaCl/150 mM KCl solution (similar to that of the cytosol) on the left (Figure 11-18a). A potentiometer (voltmeter) is connected to both solutions to measure any electric potential across the membrane. Initially, both solutions contain an equal number of positive and negative ions. If the membrane is impermeable to all ions, no ions will flow across it. Furthermore, there will be no difference in voltage, or electric potential gradient, across the membrane, as shown in Figure 11-18a.
Page 496
Now suppose that the membrane contains Na+ channels that accommodate Na+ ions but exclude K+ and Cl− ions (Figure 11-18b). Na+ ions then tend to move down their concentration gradient from the right side to the left, leaving an excess of negative Cl− ions compared with Na+ ions on the right side and generating an excess of positive Na+ ions compared with Cl− ions on the left side. The excess Na+ on the left and Cl− on the right remain near the respective surfaces of the membrane because the excess positive charges on one side of the membrane are attracted to the excess negative charges on the other side. The resulting separation of charge across the membrane constitutes an electric potential, or voltage, and the left (cytosolic) side of the membrane has excess positive charge with respect to the right.
As more and more Na+ ions move through channels across the membrane, the magnitude of this charge difference (i.e., voltage) increases. However, continued right-
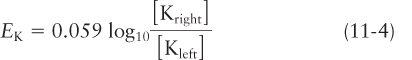
If a membrane is permeable only to Na+ ions, then at equilibrium, the measured electric potential across the membrane equals the sodium equilibrium potential in volts, ENa. The magnitude of ENa is given by the Nernst equation, which is derived from basic principles of physical chemistry:
Page 497
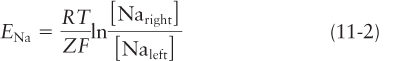
where R (the gas constant) = 1.987 cal/(degree · mol), or 8.28 joules/(degree · mol); T (the absolute temperature in degrees Kelvin) = 293 °K at 20 °C; Z (the charge, also called the valence) is here equal to +1; F (the Faraday constant) = 23,062 cal/(mol · V), or 96,000 coulombs/(mol · V); and [Naleft] and [Naright] are the Na+ concentrations on the left and right sides, respectively, at equilibrium. By convention, the potential is expressed as the cytosolic face of the membrane relative to the exoplasmic face, and the equation is written with the ion concentration of the extracellular solution (here the right side of the membrane) placed in the numerator and that of the cytosol in the denominator.
At 20 °C (room temperature), Equation 11-
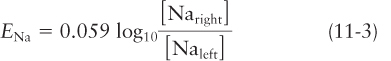
If [Naright]/[Naleft] = 10, a tenfold ratio of concentrations as in Figure 11-18b, then ENa = +0.059 V (or +59 mV), with the left, cytosolic side positive with respect to the right, exoplasmic side.
If the membrane is permeable only to K+ ions and not to Na+ or Cl− ions, then a similar equation describes the potassium equilibrium potential EK:
The magnitude of the membrane electric potential is the same (59 mV, for a tenfold difference in ion concentrations), but the left, cytosolic side is now negative with respect to the right, exoplasmic (Figure 11-18c), so the polarity is opposite to that obtained across a membrane selectively permeable to Na+ ions.