Several Cotransporters Regulate Cytosolic pH
The anaerobic metabolism of glucose yields lactic acid, and aerobic metabolism yields CO2, which combines with water to form carbonic acid (H2CO3). These weak acids dissociate, yielding H+ ions (protons); if these excess protons were not removed from cells, the cytosolic pH would drop precipitously, endangering cellular functions. Two types of cotransporters help remove some of the excess protons generated during metabolism in animal cells. One is a Na+HCO3−/Cl− antiporter, which imports one Na+ ion, together with one HCO3−, in exchange for export of one Cl− ion. The cytosolic enzyme carbonic anhydrase catalyzes the dissociation of the imported HCO3− ion into CO2 and an OH− (hydroxyl) ion:
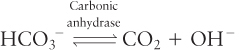
The OH− ions combine with intracellular protons, forming water, and the CO2 diffuses out of the cell. Thus the overall action of this transporter is to consume cytosolic H+ ions, thereby raising the cytosolic pH. Also important in raising cytosolic pH is a Na+/H+ antiporter, which couples the movement of one Na+ ion into the cell down its concentration gradient to the export of one H+ ion.
Under certain circumstances, the cytosolic pH can rise beyond the normal range of 7.2–
Page 506
The activity of all three of these antiporters is regulated by the cytosolic pH, providing cells with a finely tuned mechanism for controlling cytosolic pH. The two antiporters that operate to increase cytosolic pH are activated when the pH of the cytosol falls. Similarly, a rise in pH above 7.2 stimulates the Cl−/HCO3− antiporter, leading to a more rapid export of HCO3− and a drop in the cytosolic pH. In this manner, the cytosolic pH of growing cells is maintained very close to pH 7.4.