Parietal Cells Acidify the Stomach Contents While Maintaining a Neutral Cytosolic pH
The mammalian stomach contains a 0.1 M solution of hydrochloric acid (HCl). This strongly acidic medium kills many ingested pathogens and denatures many ingested proteins so that they can be degraded by proteolytic enzymes (e.g., pepsin) that function at acidic pH. Hydrochloric acid is secreted into the stomach by specialized epithelial cells called parietal cells (also known as oxyntic cells) in the stomach lining. These cells contain a H+/K+ ATPase in the apical membrane (which faces the stomach lumen) that generates a 1-
If parietal cells simply exported H+ ions in exchange for K+ ions, the loss of protons would lead to a rise in the concentration of OH− ions in the cytosol and thus a marked increase in cytosolic pH. (Recall that [H+] × [OH−] is always is a constant, 10−14 M2.) Parietal cells avoid this rise in cytosolic pH in conjunction with acidification of the stomach lumen by using Cl−/HCO3− antiporters in the basolateral membrane to export the excess OH− ions from the cytosol to the blood. As noted earlier, these anion antiporters are activated at high cytosolic pH.
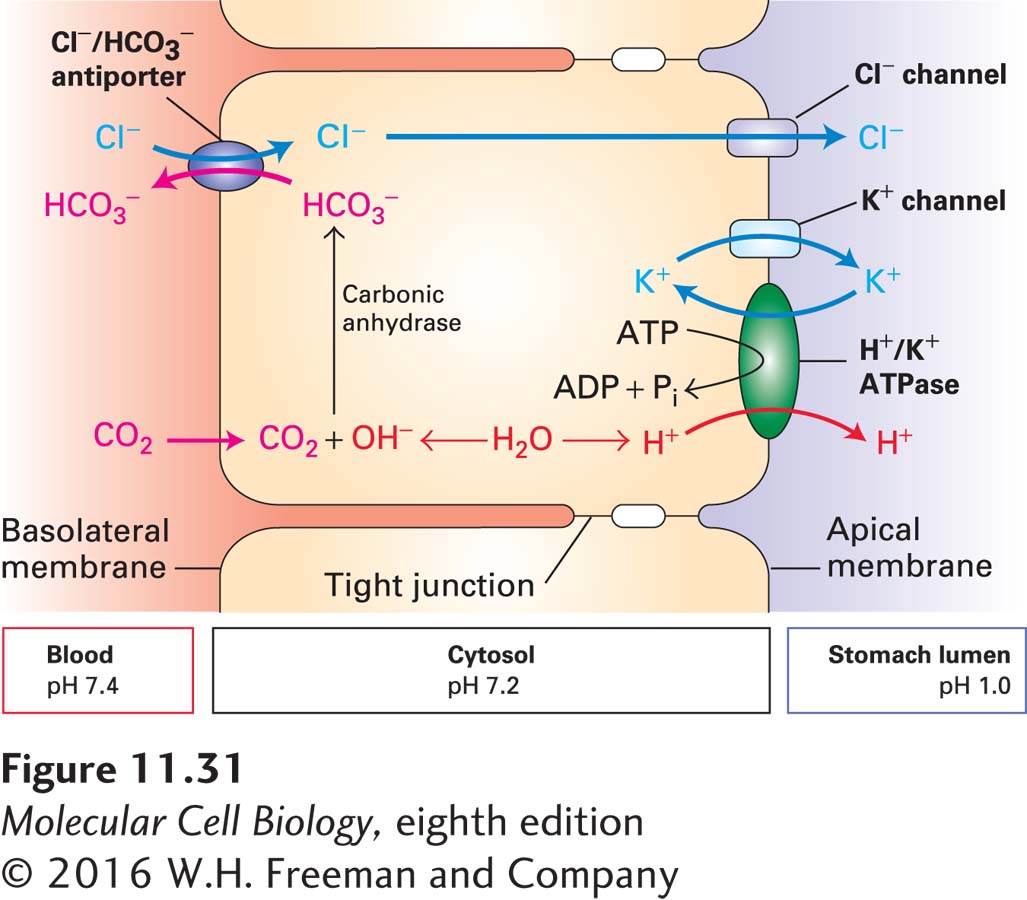
The overall process by which parietal cells acidify the stomach lumen is illustrated in Figure 11-31. In a reaction catalyzed by carbonic anhydrase, the excess cytosolic OH− combines with CO2 that diffuses in from the blood, forming HCO3−. This bicarbonate ion is exported across the basolateral membrane (and ultimately into the blood) by the Cl−/HCO3− antiporter in exchange for a Cl− ion. The Cl− ions then exit through Cl− channels in the apical membrane, entering the stomach lumen. To preserve electroneutrality, each Cl− ion that moves into the stomach lumen across the apical membrane is accompanied by a K+ ion that moves outward through a separate K+ channel. In this way, the excess K+ ions pumped inward by the H+/K+ ATPase are returned to the stomach lumen, thus maintaining the normal intracellular K+ concentration. The net result is secretion of equal amounts of H+ and Cl− ions (i.e., HCl) into the stomach lumen, while the pH of the cytosol remains neutral and the excess OH− ions, as HCO3−, are transported into the blood, where the change in pH is minimal.
Page 510