Classic Experiment 11-1
Stumbling upon Active Transport
J. Skou, 1957, Biochim. Biophys. Acta 23:394
In the mid-
Background
During the 1950s, many researchers around the world were actively investigating the physiology of the plasma membrane, which plays roles in a number of biological processes. It was well known that the concentrations of many ions differ inside and outside the cell. For example, the cell maintains a lower intracellular sodium (Na+) concentration and a higher intracellular potassium (K+) concentration than is found outside the cell. Somehow the membrane can regulate intracellular salt concentrations. Additionally, movement of ions across cellular membranes had been observed, suggesting that some sort of transport system is present. To maintain normal intracellular Na+ and K+ concentrations, that transport system could not rely on passive diffusion because both ions would have to move across the membrane against their concentration gradients. The energy-
At the time of Skou’s experiment, the mechanism of active transport was still unclear. Surprisingly, Skou had no intention of helping to clarify it. He found the Na+/K+ ATPase completely by accident in his search for an abundant, easily measured enzyme activity associated with lipid membranes. A recent study had shown that membranes derived from squid giant axons contained a membrane-
The Experiment
Since the original goal of his study was to characterize the ATPase for use in subsequent studies, Skou wanted to know under what experimental conditions its activity was both robust and reproducible. As often is the case when a new enzyme has been characterized, this requires careful titration of the various components of the reaction it catalyzes. Before such titrations can be done, however, one must be sure the system is free from outside sources of contamination.
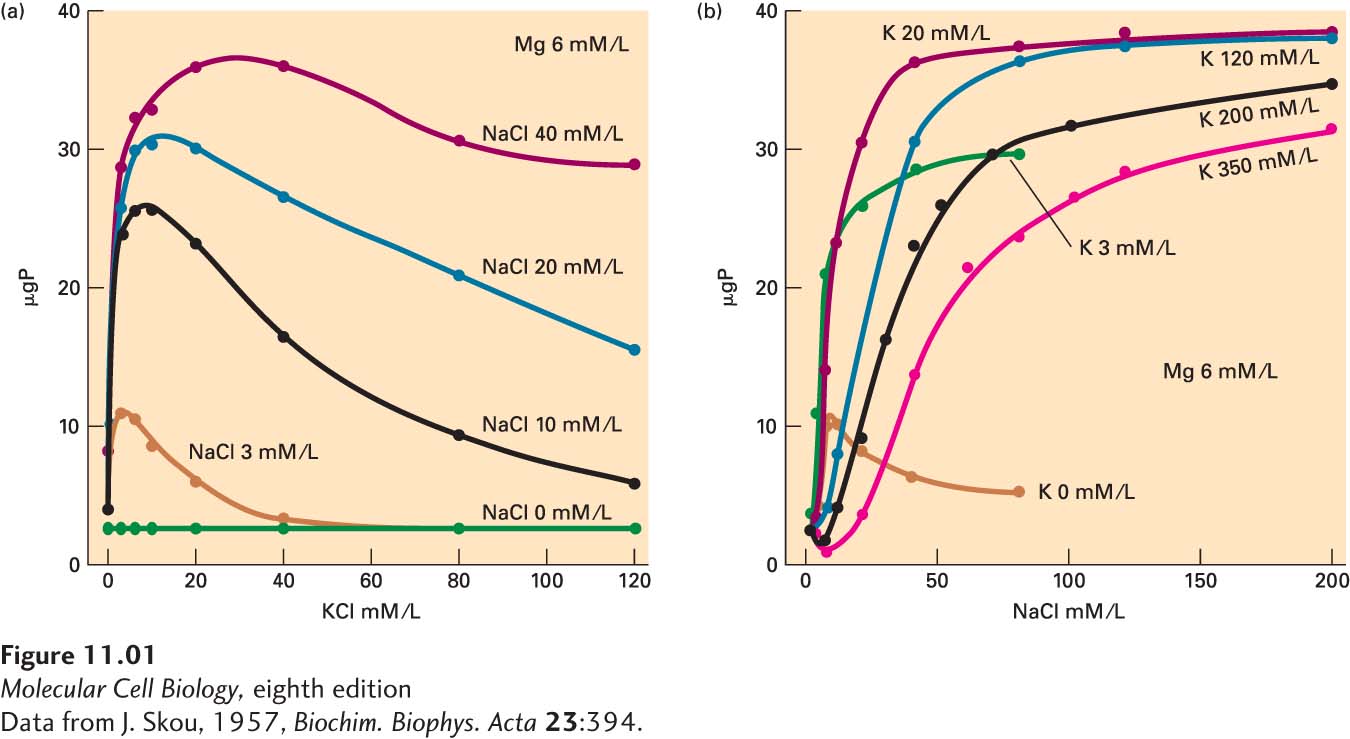
In order to study the influence of various cations, including three that are critical for the reaction—
Skou first showed that his enzyme could indeed catalyze the cleavage of ATP into ADP and inorganic phosphate. He then moved on to look for the optimal conditions for this activity by varying the pH of the reaction, as well as the concentrations of salts and other cofactors, which bring cations into the reaction. He could easily determine a pH optimum as well as an optimal concentration of Mg2+, but optimizing Na+ and K+ proved to be more difficult. Regardless of the amount of K+ added to the reaction, the enzyme was inactive without Na+. Similarly, without K+, Skou observed only a low-
These results suggested that the enzyme required both Na+ and K+ for optimal activity. To demonstrate that this was the case, Skou performed a series of experiments in which he measured the enzyme activity as he varied both the Na+ and K+ concentrations in the reaction (Figure 1). Although both cations clearly were required for significant activity, something interesting occurred at high concentrations of each cation. At the optimal concentration of Na+ and K+, the ATPase activity reached a peak. Once at that peak, further increasing the concentration did not affect the ATPase activity. Na+ thus behaved like a classic enzyme substrate, with increasing input leading to increased activity until a saturating concentration was achieved, at which the activity plateaued. K+, on the other hand, behaved differently. When the K+ concentration was increased beyond the optimum, ATPase activity declined. Thus while K+ was required for optimal activity, at high concentrations it inhibited the enzyme. Skou reasoned that the enzyme must have separate binding sites for Na+ and K+. For optimal ATPase activity, both must be filled. However, at high concentrations, K+ could compete for the Na+-binding site, leading to enzyme inhibition. He hypothesized that this enzyme was involved in active transport; that is, the pumping of Na+ out of the cell, coupled with the import of K+ into the cell. Later studies would prove that this enzyme was indeed the pump that catalyzed active transport. This finding was so exciting that Skou devoted his subsequent research to studying the enzyme, never using it as a marker as he initially intended.
Discussion
Skou’s finding that a membrane ATPase uses both Na+ and K+ as substrates was the first step in understanding active transport on a molecular level. How did Skou know to test both Na+ and K+? In his Nobel lecture in 1997, he explained that in his first attempts at characterizing the ATPase, he took no precautions to avoid the use of buffers and ATP stock solutions that contained Na+ and K+. Pondering the puzzling and irreproducible results that he obtained led to the realization that contaminating salts might be influencing the reaction. When he repeated the experiments, this time avoiding contamination by Na+ and K+ at all stages, he obtained clear-
The discovery of the Na+/K+ATPase had an enormous impact on membrane biology, leading to a better understanding of the membrane potential. The generation and disruption of membrane potential forms the basis of many biological processes, including neurotransmission and the coupling of chemical and electrical energy. For this fundamental discovery, Skou was awarded the Nobel Prize in Chemistry in 1997.