Disulfide Bonds Are Formed and Rearranged by Proteins in the ER Lumen
In Chapter 3, we learned that both intramolecular and intermolecular disulfide bonds (–S–S–) help stabilize the tertiary and quaternary structure of many proteins. These covalent bonds form by the oxidative linkage of sulfhydryl groups (–SH), also known as thiol groups, on two cysteine residues in the same or different polypeptide chains. This reaction can proceed only when a suitable oxidant is present. In eukaryotic cells, disulfide bonds are formed only in the lumen of the rough ER. Thus disulfide bonds are found only in soluble secretory proteins and in the exoplasmic domains of membrane proteins. Cytosolic proteins and organelle proteins synthesized on free ribosomes (i.e., those destined for mitochondria, chloroplasts, peroxisomes, etc.) usually lack disulfide bonds.
The efficient formation of disulfide bonds in the lumen of the ER depends on the enzyme protein disulfide isomerase (PDI), which is present in all eukaryotic cells. This enzyme is especially abundant in the ER of secretory cells in organs such as the liver and pancreas, where large quantities of proteins that contain disulfide bonds are produced. As shown in Figure 13-19a, the disulfide bond in the active site of PDI can be readily transferred to a protein by two sequential thiol-
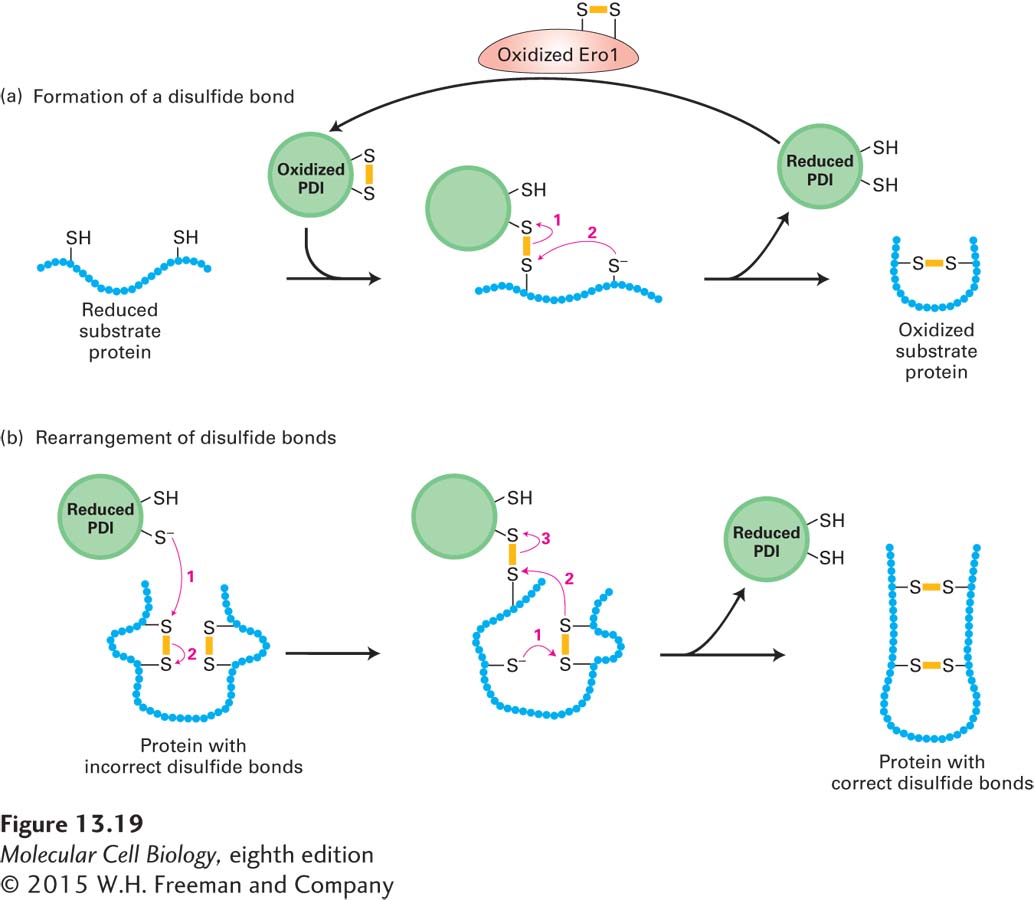
In proteins that contain more than one disulfide bond, the proper pairing of cysteine residues is essential for normal structure and activity. Disulfide bonds are commonly formed between cysteines that occur sequentially in the amino acid sequence while a polypeptide is still growing on the ribosome. Such sequential formation, however, sometimes yields disulfide bonds between the wrong cysteines. For example, proinsulin, a precursor to the peptide hormone insulin, has three disulfide bonds that link cysteines 1 and 4, 2 and 6, and 3 and 5. In this case, a disulfide bond that initially formed sequentially (e.g., between cysteines 1 and 2) would have to be rearranged for the protein to achieve its proper folded conformation. In cells, the rearrangement of disulfide bonds is also accelerated by PDI, which acts on a broad range of protein substrates, allowing them to reach their thermodynamically most stable conformations (Figure 13-19b). Disulfide bonds generally form in a specific order, first stabilizing small domains of a polypeptide, then stabilizing the interactions of more distant segments; this phenomenon is illustrated by the folding of the influenza hemagglutinin (HA) protein, discussed in the next section.
Page 604