Mitochondrial Protein Import Requires Outer-Membrane Receptors and Translocons in Both Membranes
Figure 13-24 presents an overview of protein import from the cytosol into the mitochondrial matrix, the route into the mitochondrion followed by most imported proteins. Here we discuss in detail each step of protein transport into the matrix, then consider how some proteins are subsequently targeted to other compartments of the mitochondrion.
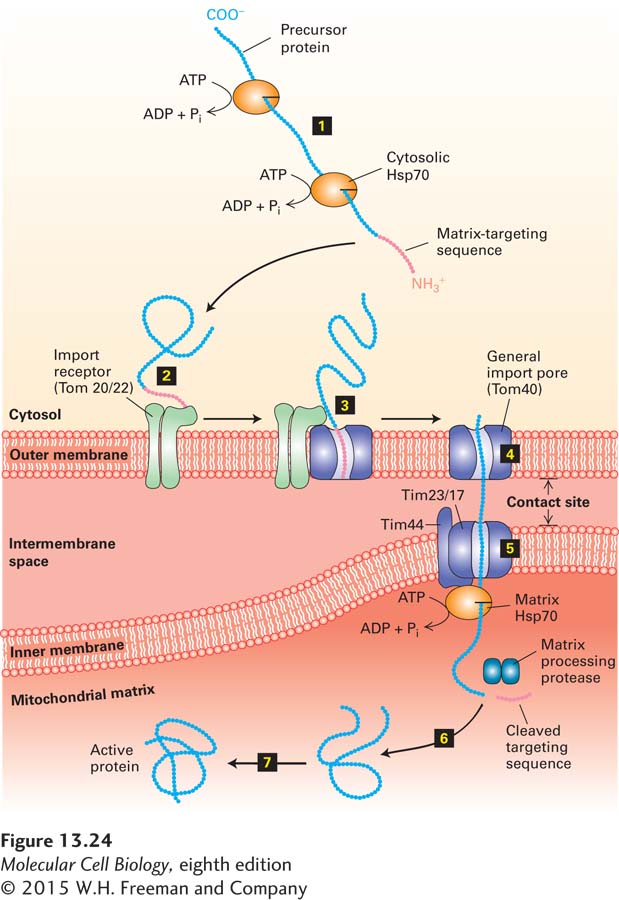
After synthesis in the cytosol, the soluble precursors of mitochondrial proteins (including hydrophobic integral membrane proteins) can interact directly with the mitochondrial membrane. Import of an unfolded mitochondrial precursor protein is initiated by the binding of a mitochondrial targeting sequence to an import receptor in the outer mitochondrial membrane. These import receptors were first identified by experiments in which antibodies to specific proteins of the outer mitochondrial membrane were shown to inhibit protein import into isolated mitochondria. Subsequent genetic experiments in which the genes for specific mitochondrial outer-
Many proteins can be imported into the mitochondrion only in an unfolded state. Chaperone proteins such as cytosolic Hsp70 and Hsp90 use energy derived from ATP hydrolysis to keep nascent and newly made proteins in a disaggregated state so that they are available to be taken up by mitochondria. For some mitochondrial precursor proteins, the mitochondrial outer-
The import receptors subsequently transfer the precursor protein to an import channel in the outer membrane. This channel, composed mainly of the Tom40 protein, is known as the general import pore because all known mitochondrial precursor proteins gain access to the interior compartments of the mitochondrion through it. When Tom40 is purified and incorporated into liposomes, it forms a transmembrane channel with a pore wide enough to accommodate an unfolded polypeptide chain. The general import pore forms a largely passive channel through the outer mitochondrial membrane; the driving force for unidirectional transport comes from within the mitochondrion, as we will see shortly. In the case of precursors destined for the mitochondrial matrix, transfer through the outer membrane occurs simultaneously with transfer through an inner-
Page 611
Soon after the N-
Some imported proteins can fold into their final, active conformation without further assistance. Final folding of many matrix proteins, however, requires chaperonins. As discussed in Chapter 3, chaperonin proteins actively facilitate protein folding by a process that depends on ATP. Yeast mutants defective in Hsc60, a chaperonin in the mitochondrial matrix, can import matrix proteins and cleave their targeting sequences normally, but the imported polypeptides fail to fold and assemble into their native tertiary and quaternary structures.
Page 612