Different G Proteins Are Activated by Different GPCRs and In Turn Regulate Different Effector Proteins
All effector proteins in GPCR signal transduction pathways are either membrane-bound ion channels or membrane-bound enzymes that catalyze the formation of one or more of the second messengers shown in Figure 15-6. The variations on the theme of GPCR signaling that we examine in Sections 15.4 through 15.6 arise because multiple G proteins with distinct activities are encoded in eukaryotic genomes. Humans have 21 different Gα subunits encoded by 16 genes, several of which undergo alternative splicing; 6 Gβ subunits; and 12 Gγ subunits. So far as is known, the different Gβγ subunits are interchangeable in their functions, while the different Gα subunits afford the various G proteins their specificity. Thus we can refer to the entire three-subunit G protein by the name of its alpha subunit.
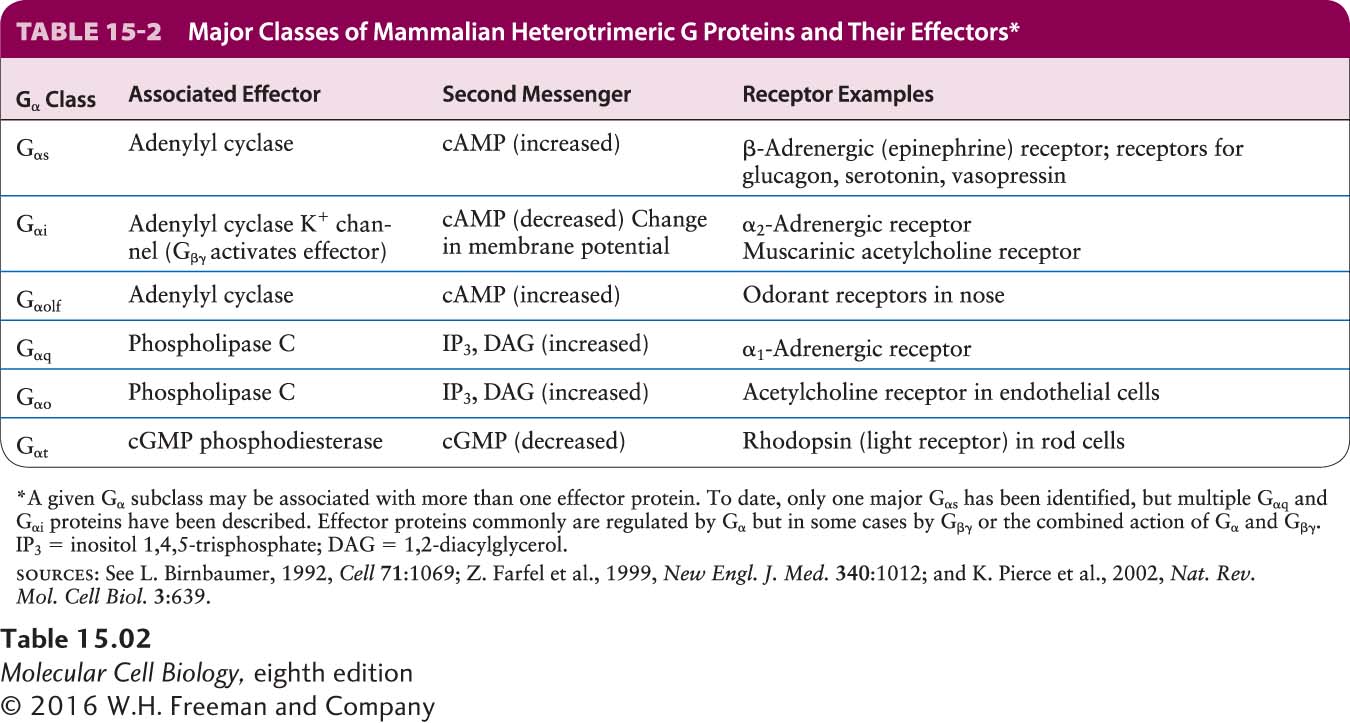
Table 15-2 summarizes the functions of the major classes of G proteins with different Gα subunits. To illustrate the versatility of these proteins, we will consider the set of G protein–coupled receptors for epinephrine found in different types of mammalian cells. The hormone epinephrine is particularly important in mediating the body’s response to stress, also known as the fight-or-flight response. As we detail in Section 15.5, during moments of fear or heavy exercise, when tissues may have an increased need to catabolize glucose and fatty acids to produce ATP, epinephrine signals for the rapid breakdown of glycogen to glucose in hepatic (liver) cells and of triacylglycerols to fatty acids in adipose (fat) cells; within seconds, these principal metabolic fuels are supplied to the blood. In mammals, the liberation of glucose and fatty acids is triggered by the binding of epinephrine (or its derivative, norepinephrine) to β2-adrenergic receptors on the surface of hepatic and adipose cells. Both subtypes of β-adrenergic receptors, termed β1 and β2, are coupled to a stimulatory G protein (Gs) whose alpha subunit (Gαs) activates a membrane-bound effector enzyme called adenylyl cyclase. Once activated, this enzyme catalyzes synthesis of the second messenger cAMP.
Page 692
Epinephrine has other physical effects as well. Epinephrine bound to β1-adrenergic receptors on heart muscle cells, for example, increases the contraction rate, which increases the blood supply to the tissues. Yet another type of epinephrine GPCR, the α1-adrenergic receptor, is found on smooth muscle cells lining the blood vessels in the intestinal tract, skin, and kidneys. Binding of epinephrine to these receptors causes the arteries to constrict, cutting off circulation to these organs. The Gαq subunit, which is coupled to the α1-adrenergic receptor, activates a different effector enzyme, phospholipase C, which generates two other second messengers, DAG and IP3 (see Figure 15-6). Yet another epinephrine receptor, the α2-adrenergic receptor, is found on many body cells and is coupled to the Gαi subunit, which causes inhibition of adenylyl cyclase in these target cells. These diverse effects of epinephrine help orchestrate integrated responses throughout the body, all directed to a common end: supplying energy to major locomotor muscles, while at the same time diverting it from other organs not as crucial in executing a response to physical stress.
 Some bacterial toxins contain a subunit that penetrates the plasma membrane of target mammalian cells and, once in the cytosol, catalyzes a chemical modification of Gα proteins that prevents hydrolysis of bound GTP to GDP. For example, toxins produced by the bacterium Vibrio cholerae, which causes cholera, or certain strains of E. coli enter intestinal epithelial cells and catalyze a covalent modification of the Gαs protein in these cells. As a result, Gαs remains in the active state, continuously activating the effector adenylyl cyclase in the absence of hormonal stimulation. The resulting excessive rise in intracellular cAMP leads to the loss of electrolytes and water into the intestinal lumen, producing the watery diarrhea characteristic of infection by these bacteria. The toxin produced by Bordetella pertussis, a bacterium that commonly infects respiratory tract cells and causes whooping cough, catalyzes a modification of Gαi that prevents release of bound GDP. As a result, Gαi is locked in the inactive state, reducing the inhibition of adenylyl cyclase. The resulting increase in cAMP in epithelial cells of the airways promotes loss of fluids and electrolytes and mucus secretion.
Some bacterial toxins contain a subunit that penetrates the plasma membrane of target mammalian cells and, once in the cytosol, catalyzes a chemical modification of Gα proteins that prevents hydrolysis of bound GTP to GDP. For example, toxins produced by the bacterium Vibrio cholerae, which causes cholera, or certain strains of E. coli enter intestinal epithelial cells and catalyze a covalent modification of the Gαs protein in these cells. As a result, Gαs remains in the active state, continuously activating the effector adenylyl cyclase in the absence of hormonal stimulation. The resulting excessive rise in intracellular cAMP leads to the loss of electrolytes and water into the intestinal lumen, producing the watery diarrhea characteristic of infection by these bacteria. The toxin produced by Bordetella pertussis, a bacterium that commonly infects respiratory tract cells and causes whooping cough, catalyzes a modification of Gαi that prevents release of bound GDP. As a result, Gαi is locked in the inactive state, reducing the inhibition of adenylyl cyclase. The resulting increase in cAMP in epithelial cells of the airways promotes loss of fluids and electrolytes and mucus secretion.
With some 800 members in total, the G protein–coupled receptors represent the largest protein family in the human genome. Approximately half of the genes encoding these proteins are thought to encode sensory receptors; of these, the majority are in the olfactory system and bind odorants. The natural ligand has not been identified for many so-called orphan GPCRs—that is, putative GPCRs without known cognate ligands. Many of these orphan receptors are likely to bind heretofore unidentified signaling molecules, including novel peptide hormones. One approach that has proved fruitful in identifying the ligands of orphan GPCRs involves expressing the gene encoding the receptor in transfected cells and using the cells as a reporter system to detect substances in tissue extracts that activate the receptor and its downstream signal transduction pathway. This approach led to the discovery of two novel peptides, termed orexin-A and orexin-B (from the Greek orexis, meaning “appetite”), that were identified as the ligands for two orphan GPCRs in the same family as the glucagon receptor. Further research showed that the orexin gene is expressed only in the hypothalamus, the part of the brain that regulates feeding. Injection of orexin into the brain ventricles of animals caused them to eat more, and expression of the orexin gene increased markedly during fasting. Both of these findings are consistent with orexin’s role in increasing appetite. Strikingly, mice deficient for orexins suffer from narcolepsy, a disorder characterized in humans by excessive daytime sleepiness (in mice, nighttime sleepiness). Moreover, very recent reports suggest that the orexin system is dysfunctional in a majority of human narcolepsy patients: orexin peptides cannot be detected in their cerebrospinal fluid (although there is no evidence of mutation in their orexin genes). These findings firmly link orexin neuropeptides and their receptors to both feeding behavior and sleep in both animals and humans.
Page 693
![]() Some bacterial toxins contain a subunit that penetrates the plasma membrane of target mammalian cells and, once in the cytosol, catalyzes a chemical modification of Gα proteins that prevents hydrolysis of bound GTP to GDP. For example, toxins produced by the bacterium Vibrio cholerae, which causes cholera, or certain strains of E. coli enter intestinal epithelial cells and catalyze a covalent modification of the Gαs protein in these cells. As a result, Gαs remains in the active state, continuously activating the effector adenylyl cyclase in the absence of hormonal stimulation. The resulting excessive rise in intracellular cAMP leads to the loss of electrolytes and water into the intestinal lumen, producing the watery diarrhea characteristic of infection by these bacteria. The toxin produced by Bordetella pertussis, a bacterium that commonly infects respiratory tract cells and causes whooping cough, catalyzes a modification of Gαi that prevents release of bound GDP. As a result, Gαi is locked in the inactive state, reducing the inhibition of adenylyl cyclase. The resulting increase in cAMP in epithelial cells of the airways promotes loss of fluids and electrolytes and mucus secretion.
Some bacterial toxins contain a subunit that penetrates the plasma membrane of target mammalian cells and, once in the cytosol, catalyzes a chemical modification of Gα proteins that prevents hydrolysis of bound GTP to GDP. For example, toxins produced by the bacterium Vibrio cholerae, which causes cholera, or certain strains of E. coli enter intestinal epithelial cells and catalyze a covalent modification of the Gαs protein in these cells. As a result, Gαs remains in the active state, continuously activating the effector adenylyl cyclase in the absence of hormonal stimulation. The resulting excessive rise in intracellular cAMP leads to the loss of electrolytes and water into the intestinal lumen, producing the watery diarrhea characteristic of infection by these bacteria. The toxin produced by Bordetella pertussis, a bacterium that commonly infects respiratory tract cells and causes whooping cough, catalyzes a modification of Gαi that prevents release of bound GDP. As a result, Gαi is locked in the inactive state, reducing the inhibition of adenylyl cyclase. The resulting increase in cAMP in epithelial cells of the airways promotes loss of fluids and electrolytes and mucus secretion.