16.2 Cytokine Receptors and the JAK/STAT Signaling Pathway
In this section and the next, we discuss two large classes of receptors that activate protein tyrosine kinases. Protein tyrosine kinases, of which there are about 90 in the human genome, phosphorylate specific tyrosine residues on target proteins, usually in the context of a specific linear sequence of amino acids in which the tyrosine is embedded. The phosphorylated target proteins can then activate one or more signaling pathways. These pathways are noteworthy because they regulate most aspects of cell proliferation, differentiation, survival, and metabolism.
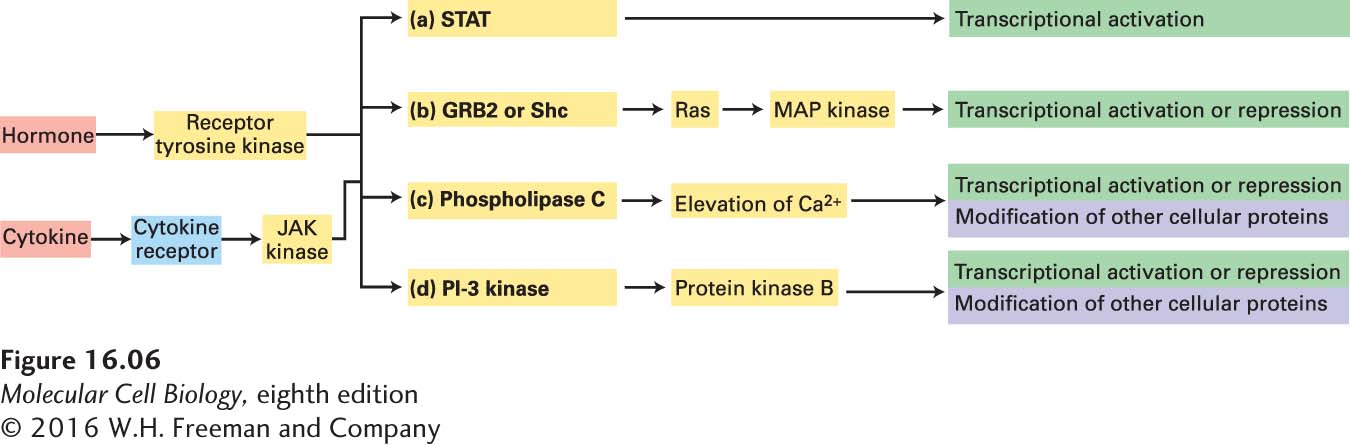
There are two broad categories of receptors that activate tyrosine kinases: (1) those in which the tyrosine kinase enzyme is an intrinsic part of the receptor’s polypeptide chain, called the receptor tyrosine kinases (RTKs), which we discuss in Section 16.3, and (2) those, such as cytokine receptors, in which the receptor and kinase are separate polypeptides, encoded by different genes, yet are bound tightly together. In cytokine receptors, the tightly bound kinase is known as a JAK kinase. Both classes of receptors activate similar intracellular signal transduction pathways (Figure 16-6). We begin with the cytokine receptors, since they mainly employ a short signal transduction pathway called the JAK/STAT pathway: a STAT transcription factor binds to the activated receptor, becomes phosphorylated by the JAK kinase, moves to the nucleus, and directly activates transcription.
Page 727