Capping Proteins Block Assembly and Disassembly at Actin Filament Ends
The treadmilling and dynamics of actin filaments are further regulated in cells by capping proteins that specifically bind to the ends of the filaments. If this were not the case, actin filaments would continue to grow and disassemble in an uncontrolled manner. As one might expect, two classes of proteins have been discovered: ones that bind the (+) end and ones that bind the (−) end (Figure 17-12).
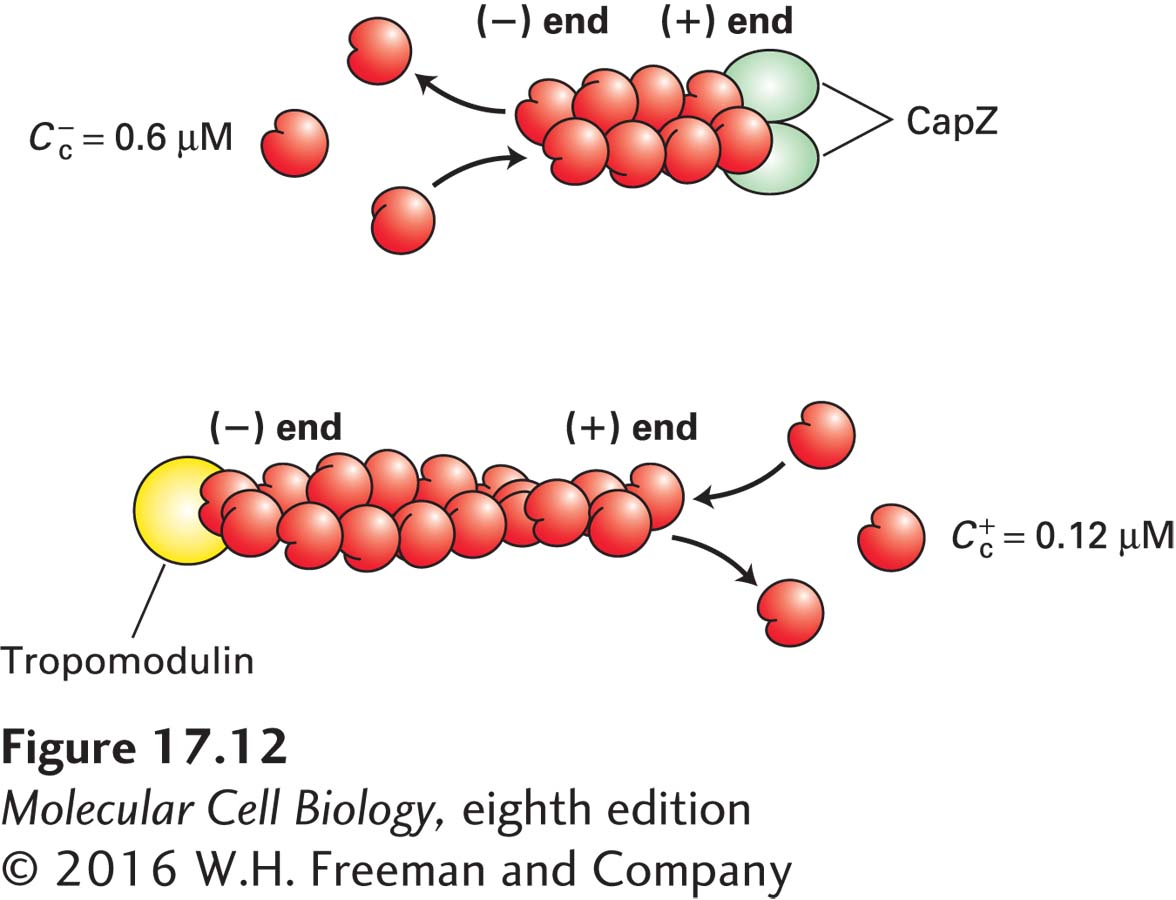
A protein known as CapZ, consisting of two closely related subunits, binds with a very high affinity (~0.1 nM) to the (+) end of an actin filament, thereby inhibiting subunit addition or loss. The concentration of CapZ in cells is generally sufficient to rapidly cap any newly formed (+) ends. So how can filaments grow at their (+) ends? At least two mechanisms regulate the activity of CapZ. First, the capping activity of CapZ is inhibited by the regulatory phospholipid phosphatidylinositol 4,5-
Another protein, called tropomodulin, binds to the (−) end of an actin filament, also inhibiting its assembly and disassembly. This protein is found predominantly in cells in which actin filaments need to be highly stabilized. Two examples of such filaments we will encounter later in this chapter are the short actin filaments in the cortex of the red blood cell and the actin filaments in muscle. In both cases, tropomodulin works with another protein, tropomyosin, which lies along the filament, to stabilize it. Tropomodulin binds to both tropomyosin and actin at the (−) end to greatly stabilize the filament.
In addition to CapZ, another class of proteins can cap the (+) ends of actin filaments. These proteins can also sever actin filaments. One member of this family, gelsolin, is regulated by Ca2+ ion concentrations. On binding Ca2+, gelsolin undergoes a conformational change that allows it to bind to the side of an actin filament and then insert itself between subunits of the helix, thereby breaking the filament. It then remains bound to and caps the (+) end, generating a new (−) end that can disassemble. As we discuss in a later section, actin cross-