Budding and Fission Yeasts Are Powerful Systems for Genetic Analysis of the Cell Cycle
Budding and fission yeasts have proved to be valuable systems for the study of the cell cycle. Although they both belong to the kingdom Fungi, they are only distantly related. Both organisms can exist in the haploid state, carrying only one copy of each chromosome. The fact that these yeasts can exist as haploid cells makes them powerful genetic systems. It is easy to generate mutations that inactivate genes in haploids because there is only one copy of each gene (a diploid would require an inactivating mutation in each of the two copies of the gene to render its activity nonfunctional). Haploid yeast can be easily employed to screen or select for mutants with specific defects, such as defects in cell proliferation. Additional advantages of these two systems are the relative ease with which one can manipulate the expression of individual genes, and the ease with which yeasts can be cultivated and manipulated so that cultures of cells progress through the cell cycle in a synchronous manner.
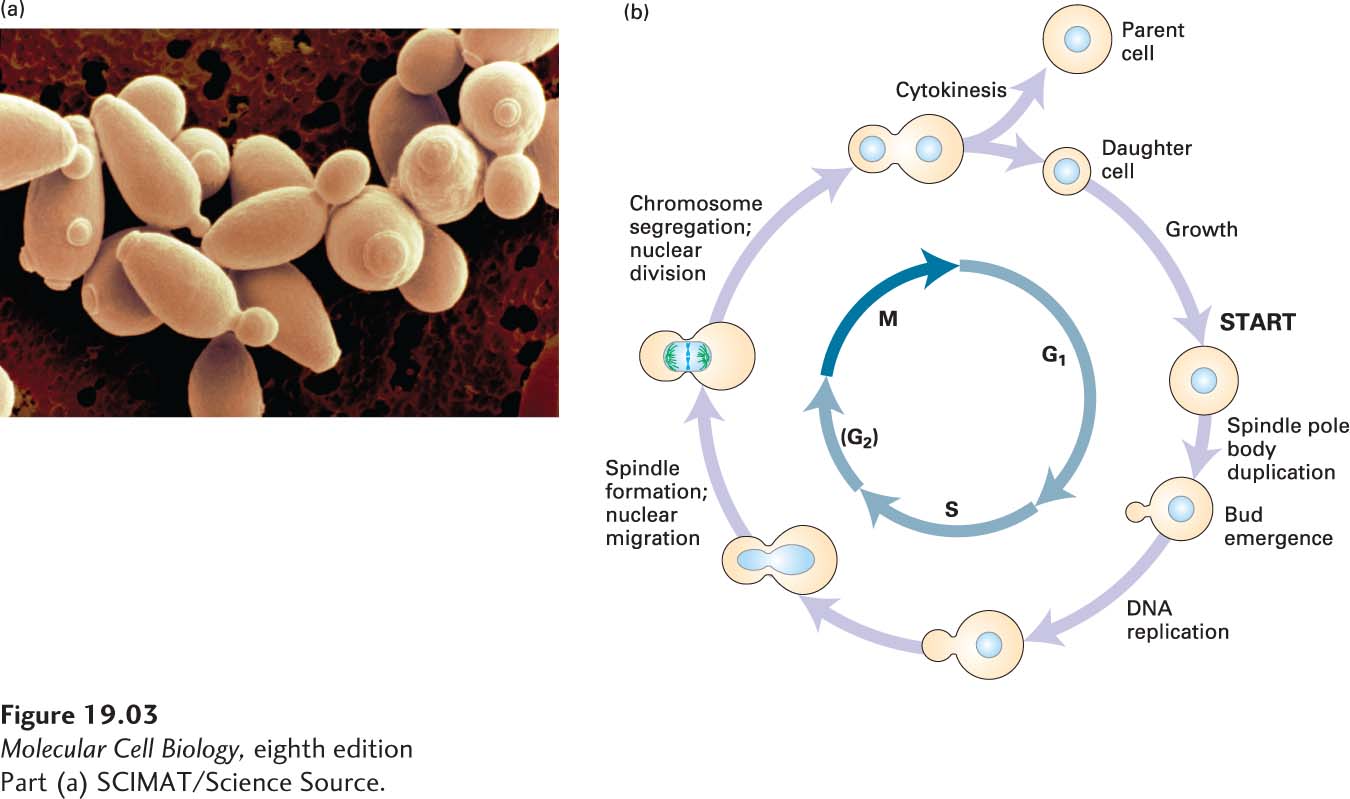
Budding yeast cells are ovoid in shape and divide by budding (Figure 19-3a). The bud, which is the future daughter cell, begins to form concomitant with the initiation of DNA replication and continues to grow throughout the cell cycle (Figure 19-3b). Cell cycle stage can therefore be inferred from the size of the bud, which makes S. cerevisiae a useful system for identifying mutants that are blocked at specific steps in the cell cycle. Indeed, it was in this organism that Lee Hartwell and colleagues first identified mutants that were defective in progressing through specific cell cycle stages. Like those of mammalian cells, the budding yeast cell cycle has a long G1 phase, and the study of the budding yeast cell cycle shaped our understanding of how the G1–S phase transition is controlled.
Page 878
Fission yeast cells are rod-
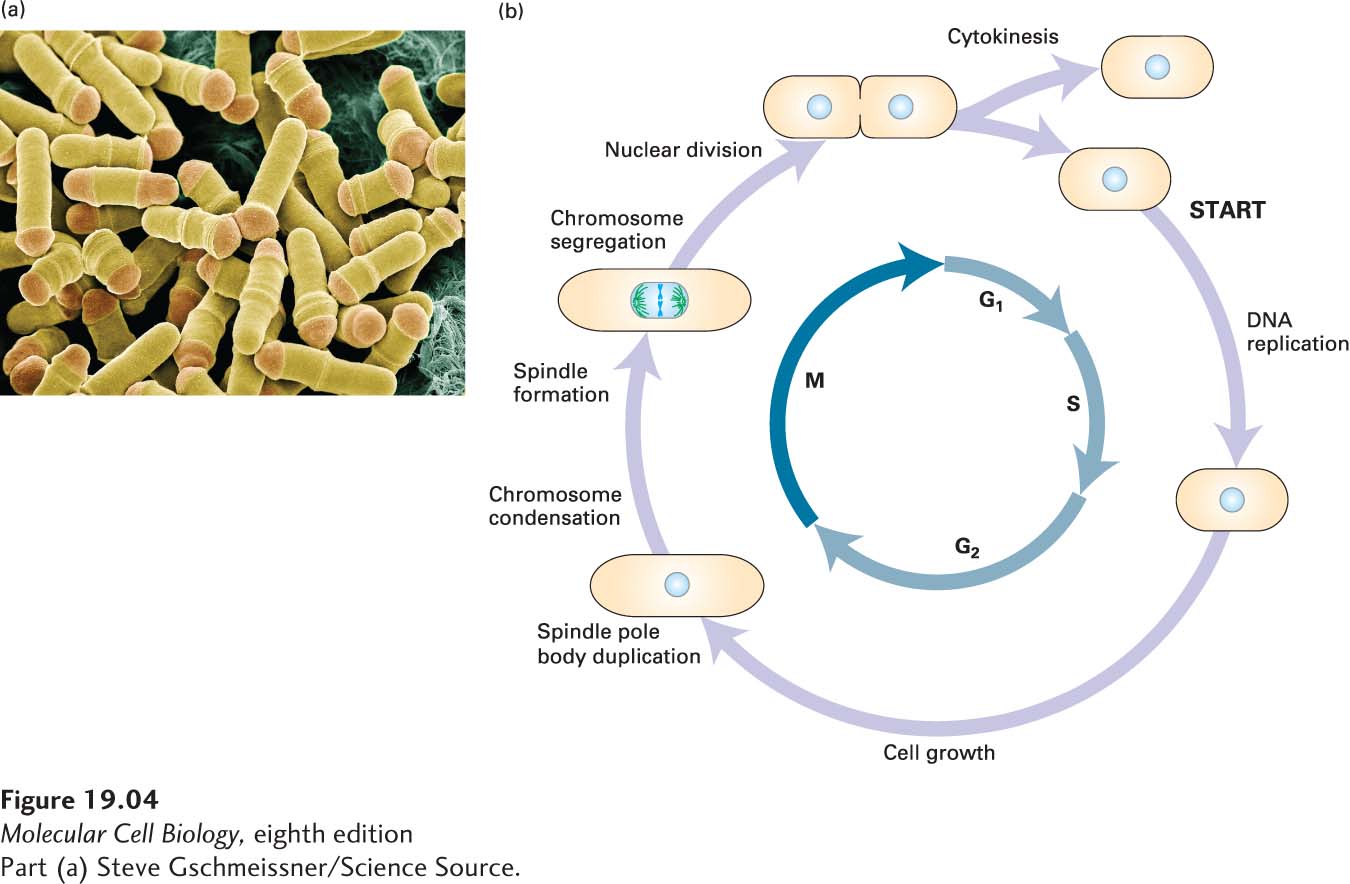
Budding and fission yeasts are both useful for the isolation of mutants that are blocked at specific steps in the cell cycle or that exhibit altered regulation of the cycle. Because cell cycle progression is essential for viability, scientists isolated conditional mutants whose genes encode proteins that are functional at one temperature but become inactive at a different, often higher, temperature (e.g., due to protein misfolding at the nonpermissive temperature; see Figure 6-6). Mutants arrested at a particular cell cycle stage are easily distinguished from normally dividing cells by microscopic examination. Thus, in both of these yeasts, cells with temperature-