Chapter Introduction
CHAPTER 2: Chemical Foundations
The life of a cell depends on thousands of chemical interactions and reactions exquisitely coordinated with one another in time and space, influenced by the cell’s genetic instructions and its environment. By understanding these interactions and reactions at a molecular level, we can begin to answer fundamental questions about cellular life: How does a cell extract nutrients and information from its environment? How does a cell convert the energy stored in nutrients into the work of movement or metabolism? How does a cell transform nutrients into the cellular components required for its survival? How does a cell link itself to other cells to form a tissue? How do cells communicate with one another so that a complex, efficiently functioning organism can develop and thrive? One of the goals of Molecular Cell Biology is to answer these and other questions about the structure and function of cells and organisms in terms of the properties of individual molecules and ions.
For example, the properties of one such molecule, water, control the evolution, structure, and function of all cells. An understanding of biology is not possible without appreciating how the properties of water control the chemistry of life. Life first arose in a watery environment. Constituting 70–
Page 32
Many of the cell’s biomolecules (such as sugars) readily dissolve in water; these molecules are referred to as hydrophilic (“water liking”). Others (such as cholesterol) are oily, fatlike substances that shun water; these molecules are said to be hydrophobic (“water fearing”). Still other biomolecules (such as phospholipids) contain both hydrophilic and hydrophobic regions; these molecules are said to be amphipathic or amphiphilic (“both liking”). The smooth functioning of cells, tissues, and organisms depends on all these molecules, from the smallest to the largest. Indeed, the chemistry of the simple proton (H+) can be as important to the survival of a human cell as that of each gigantic DNA molecule (the mass of the DNA molecule in human chromosome 1 is 8.6 × 1010 times that of a proton!). The chemical interactions of all these molecules, large and small, with water and with one another define the nature of life.
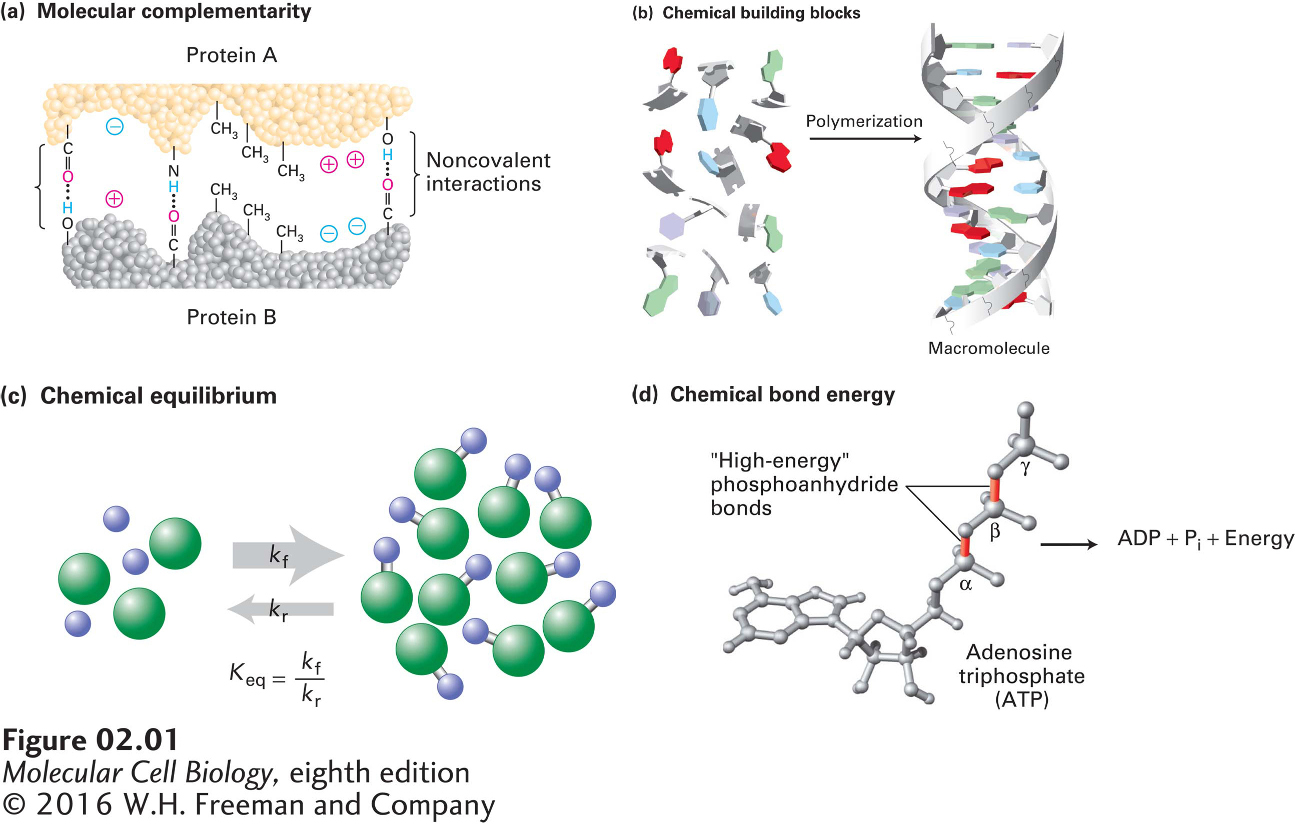
Luckily, although many types of biomolecules interact and react in numerous and complex pathways to form functional cells and organisms, a relatively small number of chemical principles are necessary to understand cellular processes at the molecular level (Figure 2-1). In this chapter, we review these key principles, some of which you already know well. We begin with the covalent bonds that connect atoms into molecules and the noncovalent interactions that stabilize groups of atoms within and between molecules. We then consider the basic chemical building blocks of macromolecules and macromolecular assemblies. After reviewing those aspects of chemical equilibrium that are most relevant to biological systems, we end the chapter with the basic principles of biochemical energetics, including the central role of ATP (adenosine triphosphate) in capturing and transferring energy in cellular metabolism.