The Equilibrium Constant Reflects the Extent of a Chemical Reaction
For any chemical reaction, Keq depends on the chemical nature of the reactants and products, the temperature, and the pressure (particularly in reactions involving gases). Under standard physical conditions (25 °C and 1 atm pressure for biological systems), Keq is always the same for a given reaction, whether or not a catalyst is present.
For the general reaction with three reactants and three products,

where capital letters represent particular molecules or atoms and lowercase letters represent the number of each in the reaction, the formula for the equilibrium constant is given by
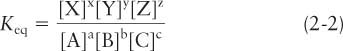
where brackets denote the concentrations of the molecules. In Equation 2-
Rateforward = kf[A]a[B]b[C]c
where kf is the rate constant for the forward reaction. Similarly, the rate of the reverse reaction (right to left in Equation 2-
Ratereverse = kr[X]x[Y]y[Z]z
where kr is the rate constant for the reverse reaction. These reaction rate equations apply whether or not the reaction has reached equilibrium. It is important to remember that the forward and reverse rates of a reaction can change because of changes in reactant or product concentrations, yet at the same time the forward and reverse rate constants do not change; hence the name “constant.” Confusing rates and rate constants is a common error. At equilibrium the forward and reverse rates are equal, so Rateforward/Ratereverse = 1. By rearranging these equations, we can express the equilibrium constant as the ratio of the rate constants:

The concept of Keq is particularly helpful when we want to think about the energy that is released or absorbed when a chemical reaction occurs. We will discuss this concept in considerable detail in Section 2.4.