Hydrogen Ions Are Released by Acids and Taken Up by Bases
In general, an acid is any molecule, ion, or chemical group that tends to release a hydrogen ion (H+), such as the carboxyl group (–COOH), which tends to dissociate to form the negatively charged carboxylate ion (–COO–); or hydrochloric acid (HCl). Conversely, a base is any molecule, ion, or chemical group that readily combines with an H+, such as the hydroxyl ion (OH–); ammonia (NH3), which forms an ammonium ion (NH4+); or the amino group (–NH2).
When an acid is added to an aqueous solution, the [H+] increases, and the pH goes down. Conversely, when a base is added to a solution, the [H+] decreases, and the pH goes up. Because [H+][OH–] = 10–14 M2, any increase in [H+] is coupled with a commensurate decrease in [OH–], and vice versa.
Many biological molecules contain both acidic and basic groups. For example, in neutral solutions (pH = 7.0), many amino acids exist predominantly in the doubly ionized form, in which the carboxyl group has lost a proton and the amino group has accepted one:

where R represents the uncharged side chain. Such a molecule, containing an equal number of positive and negative ions, is called a zwitterion. Zwitterions, having no net charge, are neutral. At extreme pH values, only one of these two ionizable groups of an amino acid is charged: the –NH2+ at low pH and the –COO– at high pH.
The dissociation reaction for an acid (or acid group in a larger molecule) HA can be written as HA ⇌ H+ + A–. The equilibrium constant for this reaction, denoted Ka (the subscript a stands for “acid”), is defined as Ka = [H+][A–]/[HA]. Taking the logarithm of both sides and rearranging the result yields a very useful relation between the equilibrium constant and pH:
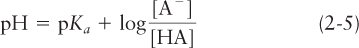
where pKa equals –log Ka.
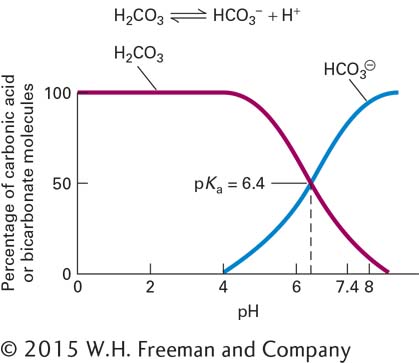
From this expression, commonly known as the Henderson-