The Change in Free Energy Determines If a Chemical Reaction Will Occur Spontaneously
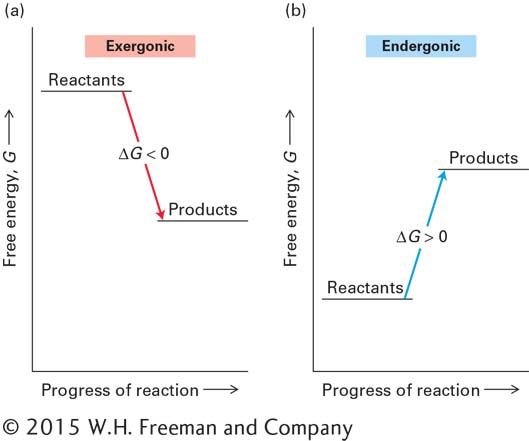
Chemical reactions can be divided into two types, depending on whether energy is absorbed or released in the process. In an exergonic (“energy-
A fundamentally important concept in understanding if a reaction is exergonic or endergonic, and therefore if it occurs spontaneously or not, is free energy (G), or Gibbs free energy, named after J. W. Gibbs. Gibbs, who received the first PhD in engineering in America in 1863, showed that “all systems change in such a way that free energy [G] is minimized.” In other words, a chemical reaction occurs spontaneously when the free energy of the products is lower than the free energy of the reactants. In the case of a chemical reaction, reactants ⇌ products, the free-
ΔG = Gproducts – Greactants
The relation of ΔG to the direction of any chemical reaction can be summarized in three statements:
If ΔG is negative, the forward reaction will tend to occur spontaneously, and energy usually will be released as the reaction takes place (exergonic reaction) (Figure 2-29). A reaction with a negative ΔG is referred to as thermodynamically favorable.
If ΔG is positive, the forward reaction will not occur spontaneously; energy will have to be added to the system in order to force the reactants to become products (endergonic reaction).
If ΔG is zero, both forward and reverse reactions will occur at equal rates, and there will be no spontaneous net conversion of reactants to products, or vice versa; the system is at equilibrium.
By convention, the standard free-
Page 59
The free energy of a chemical system can be defined as G = H – TS, where H is the bond energy, or enthalpy, of the system; T is its temperature in degrees Kelvin (K); and S is the entropy, a measure of its randomness or disorder. According to the second law of thermodynamics, the natural tendency of any isolated system is to become more disordered—
ΔG = ΔH – TΔS (2-
In an exothermic (“heat-
Many biological reactions lead to an increase in order and thus a decrease in entropy (ΔS < 0). An obvious example is the reaction that links amino acids to form a protein. A solution of protein molecules has a lower entropy than does a solution of the same amino acids unlinked because the free movement of any amino acid is more restricted (greater order) when it is bound into a long chain than when it is not. Thus, when cells synthesize polymers such as proteins from their constituent monomers, the polymerizing reaction will be spontaneous only if the cells can efficiently transfer energy to both generate the bonds that hold the monomers together and overcome the loss in entropy that accompanies polymerization. Often cells accomplish this feat by “coupling” such synthetic, entropy-
The actual change in free energy during a reaction is influenced by temperature, pressure, and the initial concentrations of reactants and products, so it usually differs from the standard free-
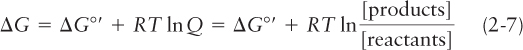
where R is the gas constant of 1.987 cal/(degree·mol), T is the temperature (in degrees Kelvin), and Q is the initial ratio of products to reactants. For a reaction A + B ⇌ C, in which two molecules combine to form a third, Q in Equation 2-
Regardless of the ΔG°′ of a particular biochemical reaction, it will proceed spontaneously within cells only if ΔG is negative given the intracellular concentrations of reactants and products. For example, the conversion of glyceraldehyde 3-
G3P ⇌ DHAP
has a ΔG°′ of –1840 cal/mol. If the initial concentrations of G3P and DHAP are equal, then ΔG = ΔG°′ because RT ln = 0; in this situation, the reversible reaction G3P ⇌ DHAP will proceed spontaneously in the direction of DHAP formation until equilibrium is reached. However, if the initial [DHAP] is 0.1 M and the initial [G3P] is 0.001 M, with other conditions standard, then Q in Equation 2-
Page 60
The ΔG of a reaction is independent of the reaction rate. Indeed, under normal physiological conditions, few, if any, of the biochemical reactions needed to sustain life would occur without some mechanism for increasing reaction rates. As we describe below and in more detail in Chapter 3, the rates of reactions in biological systems are usually determined by the activity of enzymes, the protein catalysts that accelerate the formation of products from reactants without altering the value of ΔG.