Van der Waals Interactions Are Weak Attractive Interactions Caused by Transient Dipoles
When any two atoms approach each other closely, they create a weak, nonspecific attractive force called a van der Waals interaction. These nonspecific interactions result from the momentary random fluctuations in the distribution of the electrons of any atom, which give rise to a transient unequal distribution of electrons. If two noncovalently bonded atoms are close enough, electrons of one atom will perturb the electrons of the other. This perturbation generates a transient dipole in the second atom, and the two dipoles attract each other weakly (Figure 2-10). Similarly, a polar covalent bond in one molecule attracts an oppositely oriented dipole in another.
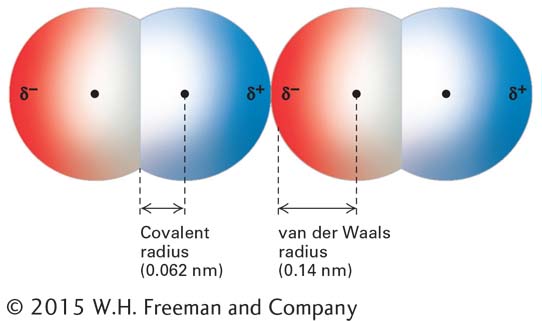
Van der Waals interactions, involving either transient or permanent dipoles, occur in all types of molecules, both polar and nonpolar. In particular, van der Waals interactions are responsible for the cohesion between nonpolar molecules such as heptane, CH3–(CH2)5–CH3, that cannot form hydrogen bonds or ionic interactions with each other. The strength of van der Waals interactions decreases rapidly with increasing distance; thus these noncovalent interactions can form only when atoms are quite close to one another. However, if atoms get too close together, the negative charges of their electrons create a repulsive force. When the van der Waals attraction between two atoms exactly balances the repulsion between their two electron clouds, the atoms are said to be in van der Waals contact. The strength of the van der Waals interaction is about 1 kcal/mol, so it is weaker than typical hydrogen bonds, and its energy is only slightly higher than the average thermal energy of molecules at 25 °C. Thus multiple van der Waals interactions, a van der Waals interaction together with other noncovalent interactions, or both are required to form van der Waals–
Page 39