Review the Concepts
1. The three-
2. Proper folding of proteins is essential for their biological activity. In general, the functional conformation of a protein is the conformation with lowest energy. This means that if an unfolded protein is allowed to reach equilibrium, it should assemble automatically into its native, functioning folded state. Why then is there a need for molecular chaperones and chaperonins in cells? What different roles do molecular chaperones and chaperonins play in the folding of proteins?
3. Enzymes catalyze chemical reactions. What constitutes the active site of an enzyme? What are the turnover number (kcat), the Michaelis constant (Km), and the maximal velocity (Vmax) of an enzyme? The kcat (catalytic rate constant) for carbonic anhydrase is 5 × 105 molecules per second. This is a “rate constant,” but not a “rate.” What is the difference? By what concentration would you multiply this rate constant in order to determine an actual rate of product formation (V)? Under what circumstances would this rate become equal to the maximal velocity (Vmax) of the enzyme?
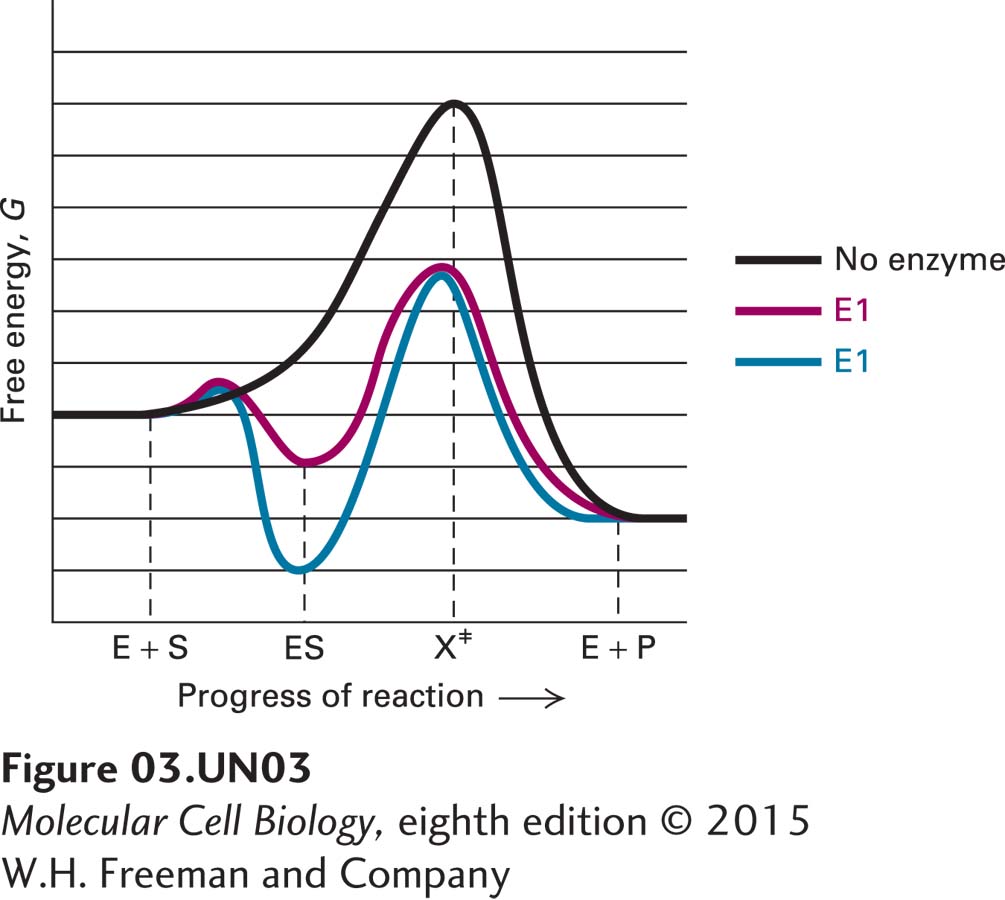
4. The following reaction coordinate diagram charts the energy of a substrate molecule (S) as it passes through a transition state (X‡) on its way to becoming a stable product (P) alone or in the presence of one of two different enzymes (E1 and E2). How does the addition of either enzyme affect the change in Gibbs free energy (ΔG) for the reaction? Which of the two enzymes binds with greater affinity to the substrate? Which enzyme better stabilizes the transition state? Which enzyme functions as a better catalyst?
Page 126
5. A healthy immune system can raise antibodies that recognize and bind with high affinity to almost any stable molecule. The molecule to which an antibody binds is known as an antigen. Antibodies have been exploited by enterprising scientists to generate valuable tools for research, diagnosis, and therapy. One clever application is the generation of antibodies that function like enzymes to catalyze complicated chemical reactions. If you wished to produce such a “catalytic” antibody, what would you suggest using as the antigen? Should it be the substrate of the reaction? The product? Something else?
6. Proteins are degraded in cells. What is ubiquitin, and what role does it play in tagging proteins for degradation? What is the role of proteasomes in protein degradation? How might proteasome inhibitors serve as chemotherapeutic (cancer-
7. The function of proteins can be regulated in a number of ways. What is cooperativity, and how does it influence protein function? Describe how protein phosphorylation and proteolytic cleavage can modulate protein function.
8. A number of techniques can separate proteins on the basis of their differences in mass. Describe the use of two of these techniques, centrifugation and gel electrophoresis. The blood proteins transferrin (MW 76 kDa) and lysozyme (MW 15 kDa) can be separated by rate-
9. Liquid chromatography is an analytical method used to separate proteins. Describe the principles for separating proteins by gel filtration, ion-
10. Various methods have been developed for detecting proteins. Describe how radioisotopes and autoradiography can be used for labeling and detecting proteins. How does Western blotting detect proteins?
11. Physical methods are often used to determine protein conformation. Describe how x-
12. Mass spectrometry is a powerful tool in proteomics. What are the four key features of a mass spectrometer? Describe briefly how MALDI and two-