Duplex DNA Is Unwound, and Daughter Strands Are Formed at the DNA Replication Fork
In order for duplex DNA to function as a template during replication, the two intertwined strands must be unwound, or melted, to make their bases available for pairing with the bases of the dNTPs that are polymerized into the newly synthesized daughter strands. This unwinding of the parent DNA strands is performed by enzymes called helicases. Unwinding begins at segments in a DNA molecule called replication origins, or simply origins. The nucleotide sequences of origins from different organisms vary greatly, although they usually contain AT-
The DNA region at which all these proteins come together to carry out the synthesis of daughter strands is called the replication fork. As replication proceeds, the replication fork and the associated proteins move away from the origin. As noted earlier, local unwinding of duplex DNA produces torsional stress, which is relieved by topoisomerase I. In order for DNA polymerases to move along and copy a duplex DNA, helicase must sequentially unwind the duplex and topoisomerase must remove the supercoils that form.
A major complication in the operation of a DNA replication fork arises from two properties of DNA: the two strands of the parent DNA duplex are antiparallel, and DNA polymerases (like RNA polymerases) can add nucleotides to the growing daughter strands only in the 5′→3′ direction. Synthesis of one daughter strand, called the leading strand, can proceed continuously from a single RNA primer in the 5′→3′ direction, the same direction as movement of the replication fork (Figure 5-29). The problem comes in synthesis of the other daughter strand, called the lagging strand.
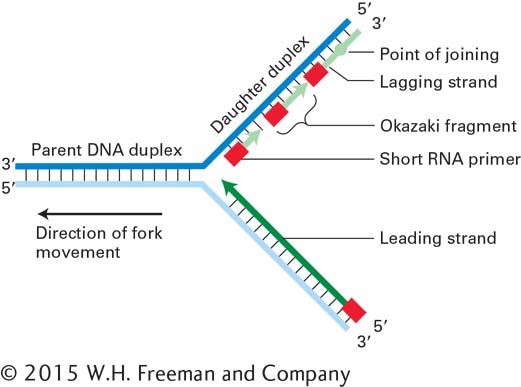
Because growth of the lagging strand must occur in the 5′→3′ direction, copying of its template strand must somehow proceed in the opposite direction from the movement of the replication fork. A cell accomplishes this feat by synthesizing a new primer every 100 to 200 nucleotides on that template strand as more of the strand is exposed by unwinding. Each of these primers, base-