DNA Can Undergo Reversible Strand Separation
During replication and transcription of DNA, the strands of the double helix must separate to allow the internal edges of the bases to pair with the bases of the nucleotides being polymerized into new complementary polynucleotide chains. In later sections, we describe the cellular mechanisms that separate and subsequently reassociate DNA strands during replication and transcription. Here we discuss the fundamental factors that influence the separation and reassociation of DNA strands. These properties of DNA were elucidated by in vitro experiments.
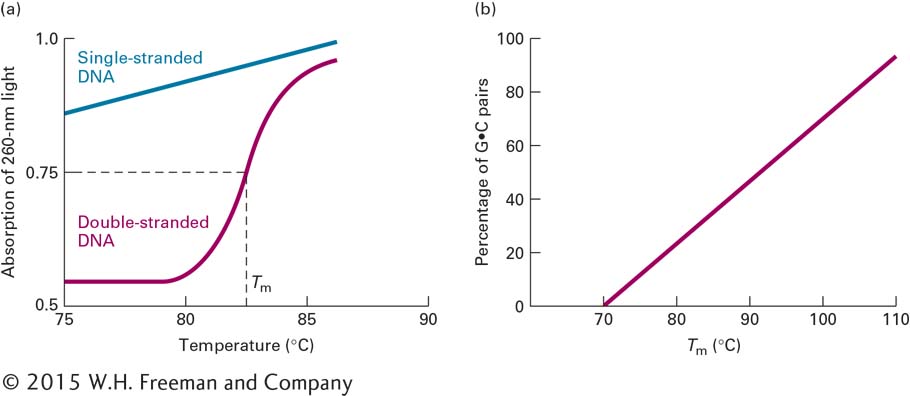
The unwinding and separation of DNA strands, referred to as denaturation, or melting, can be induced experimentally by increasing the temperature of a solution of DNA. As the thermal energy increases, the resulting increase in molecular motion eventually breaks the hydrogen bonds and other forces that stabilize the double helix. The strands then separate, driven apart by the electrostatic repulsion of the negatively charged deoxyribose-
The melting temperature (Tm) at which DNA strands separate depends on several factors. Molecules that contain a greater proportion of G·C pairs require higher temperatures to denature because the three hydrogen bonds in G·C pairs make these base pairs more stable than A·T pairs, which have only two hydrogen bonds. Indeed, the percentage of G·C base pairs in a DNA sample can be estimated from its Tm (Figure 5-7b). The ion concentration of the solution also influences the Tm because the negatively charged phosphate groups in the two strands are shielded by positively charged ions. When the ion concentration is low, this shielding is decreased, thus increasing the repulsive forces between the strands and reducing the Tm. Agents that destabilize hydrogen bonds, such as formamide or urea, also lower the Tm. Finally, extremes of pH denature DNA at low temperatures. At low (acid) pH, the bases become protonated and thus positively charged, repelling each other. At high (alkaline) pH, the bases lose protons and become negatively charged, again repelling each other because of their similar charges. In cells, pH and temperature are, for the most part, maintained at a constant level. These features of DNA denaturation are most useful for manipulating DNA in a laboratory setting.
Page 173
The single-
Page 174