
Chapter 3. Two-Dimensional Gel Electrophoresis
Introduction

Analyze the Data 3-2: Two-Dimensional Gel Electrophoresis
Proteomics involves the global analysis of protein expression. In one approach, all the proteins in control cells and in treated cells are extracted and subsequently separated using two-dimensional gel electrophoresis. Typically, hundreds or thousands of protein spots are resolved, and the steady-state levels of each protein are compared between control and treated cells. In the following example, only a few protein spots are shown for simplicity. Proteins are separated in the first dimension on the basis of charge by isoelectric focusing (pH 4–10) and then separated by size by SDS-PAGE. Proteins are detected with a stain such as Coomassie blue and assigned numbers for identification.
a. Cells are treated with a drug (“+ Drug”) or left untreated (“Control”). Proteins are then extracted and separated by two-dimensional gel electrophoresis. The stained gels are shown below.
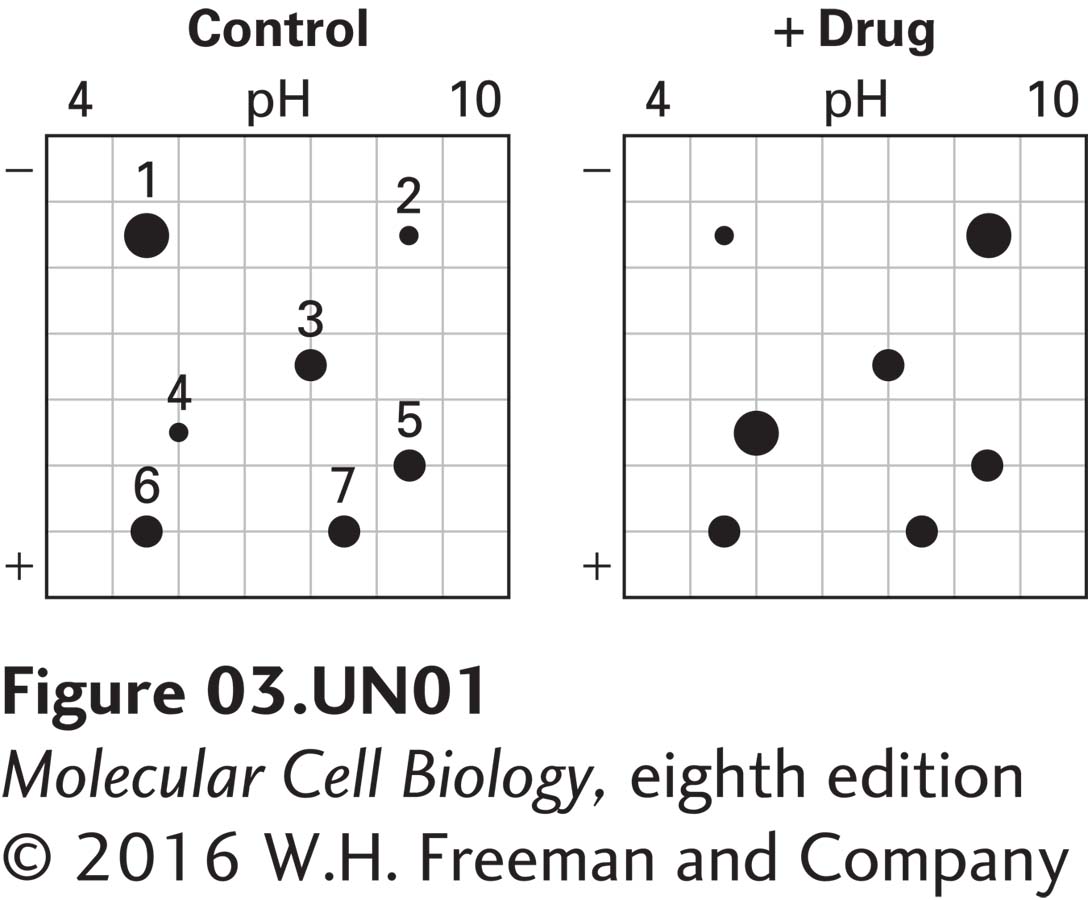
What do you conclude about the effect of the drug on the steady-state levels of proteins 1–7?
b. You suspect that the drug may be inducing a protein kinase and so repeat the experiment in part (a) in the presence of 32P-labeled inorganic phosphate. In this experiment, the two-dimensional electrophoresis gels are exposed to x-ray film to detect the presence of 32P-labeled proteins. The x-ray films are shown below.
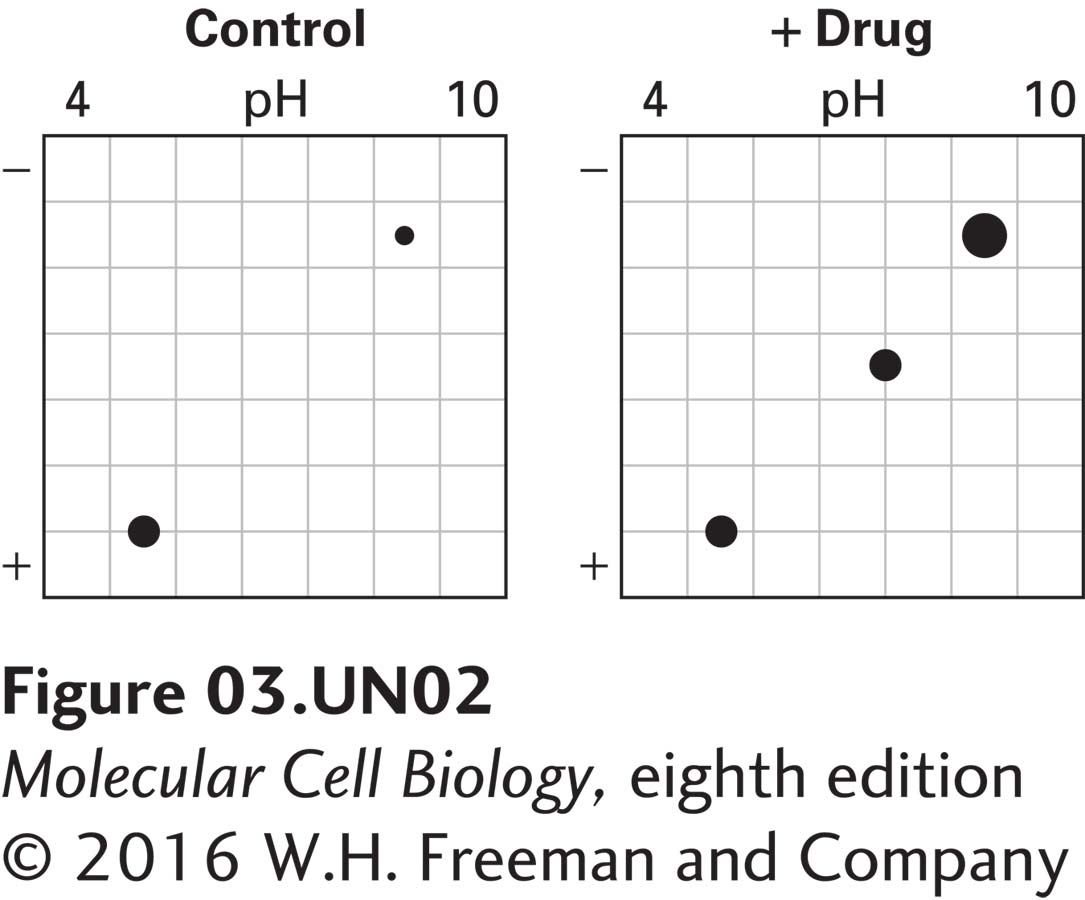
What do you conclude from this experiment about the effect of the drug on proteins 1–7?
c. To determine the cellular localization of proteins 1–7, control and treated cells from part (a) are separated into nuclear and cytoplasmic fractions by differential centrifugation. Two-dimensional electrophoresis gels are run on each fraction. The stained gels are shown below.
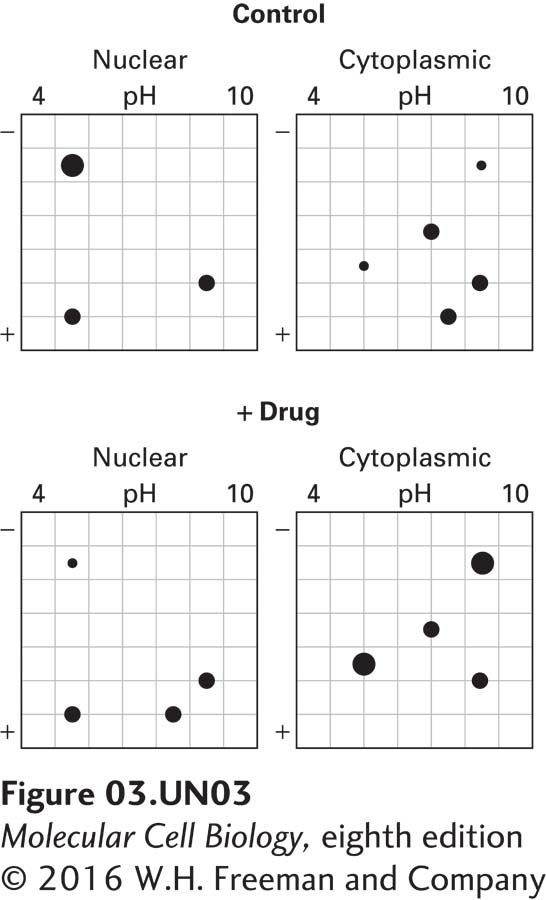
What do you conclude about the cellular localization of proteins 1–7?
d. Summarize the overall properties of proteins 1–7, combining the data from parts (a), (b), and (c). Describe how you could determine the identity of any one of the proteins.
Activity results are being submitted...