
Chapter 7. Investigating the Function of a Transmembrane Protein
Introduction

Analyze the Data 7-1: Investigating the Function of a Transmembrane Protein
The behavior of receptor X (XR), a transmembrane protein present in the plasma membrane of mammalian cells, is being investigated. The protein has been engineered as a fusion protein containing green fluorescent protein (GFP) at its N-terminus. GFP-XR is a functional protein and can replace XR in cells.
a. Cells expressing GFP-XR and artificial lipid vesicles (liposomes) containing GFP-XR are subjected to fluorescence recovery after photobleaching (FRAP). The intensity of the fluorescence of a small spot on the surface of the cells (solid line) or on the surface of the liposomes (dashed line) is measured before and after laser bleaching (arrow). The results are shown below.
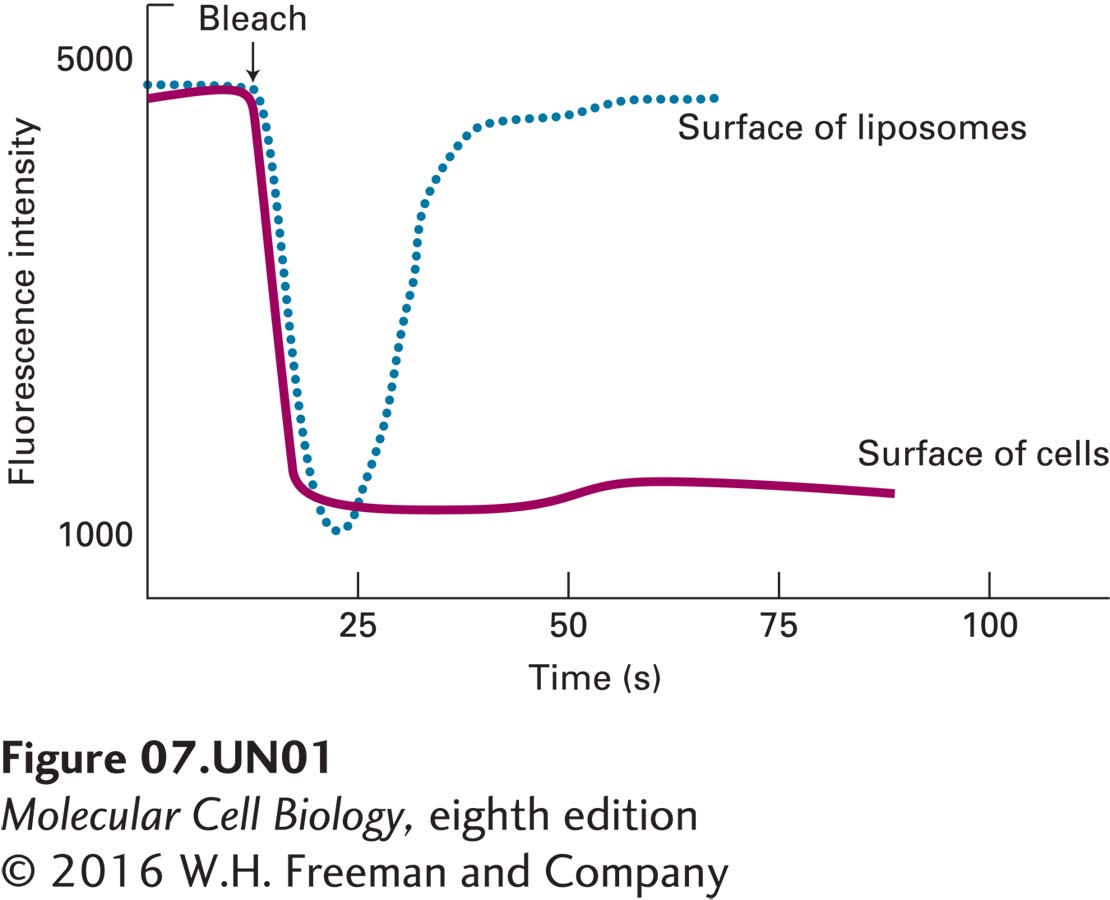
What explanation could account for the differing behavior of GFP-XR in liposomes and in the plasma membranes of cells?
b. Tiny gold particles can be attached to individual molecules and their movement then followed in a light microscope by single-particle tracking. This method allows one to observe the behavior of individual proteins in a membrane. The tracks generated during a 5-second observational period by a gold particle attached to XR present in a cell (left), to XR present in a liposome (middle), and to XR adhered to a microscope slide (right) are shown below.

What additional information do these data provide beyond what can be determined from the FRAP data?
c. Förster resonance energy transfer (FRET) is a technique that relies on the ability of a fluorescent molecule, following its excitation with the appropriate wavelength of light, to transfer its emission energy to and excite a nearby, different fluorescent molecule (see Figure 4-24). Cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) are related to GFP, but fluoresce at cyan and yellow wavelengths rather than at green. If CFP is excited with the appropriate wavelength of light and a YFP molecule is very near, then energy can be transferred from CFP emission and used to excite YFP, as indicated by a loss of emission of cyan fluorescence and an increase in emission of yellow fluorescence. CFP-XR and YFP-XR are expressed together in a cell line or are both incorporated into liposomes. The number of molecules of YFP-XR and CFP-XR per square centimeter of membrane is equivalent in the cells and in the liposomes. The cells and liposomes are then irradiated with a wavelength of light that causes CFP, but not YFP, to fluoresce. The amounts of cyan (CFP) and yellow (YFP) fluorescence emitted by the cells (solid line) and by the liposomes (dashed line) is then monitored, as shown below.
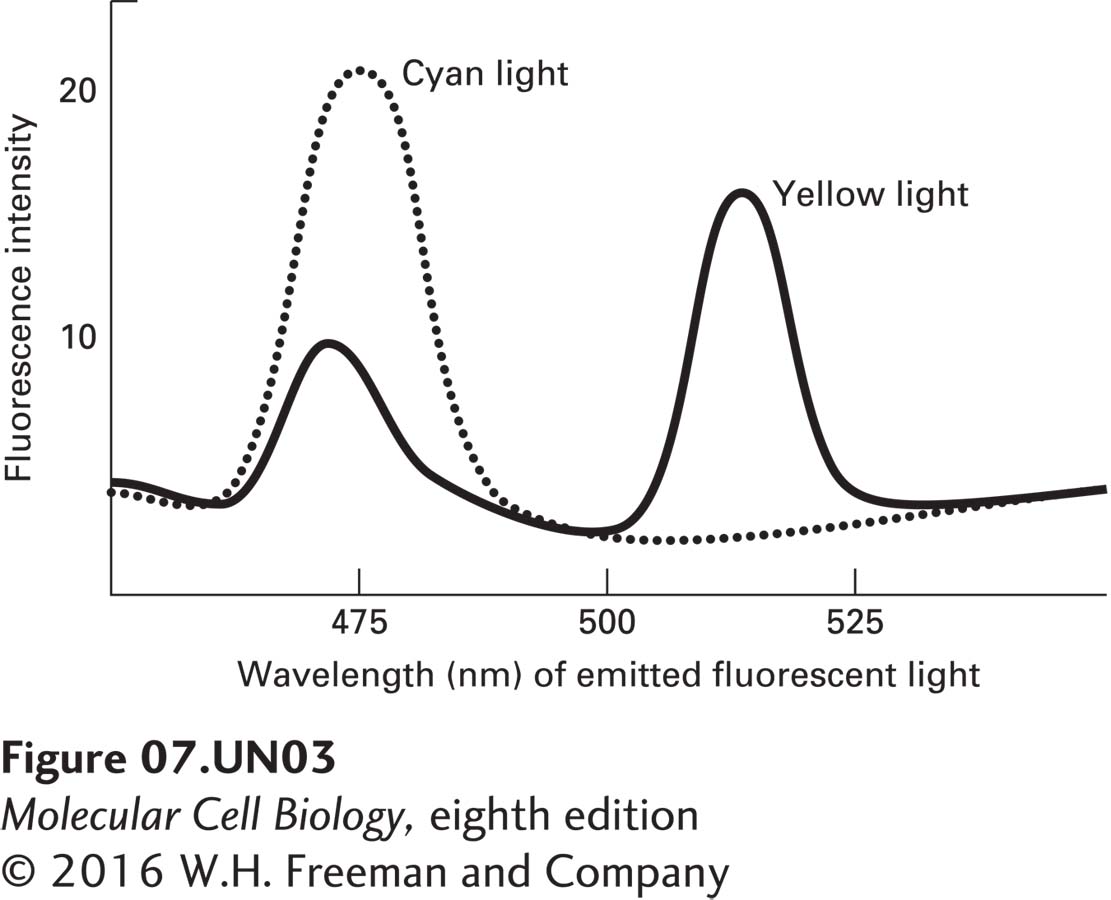
What can be deduced about XR from these data?
Activity results are being submitted...