
Chapter 12. The Mitochondrial pH Gradient
Introduction

Analyze the Data 12-1: The Mitochondrial pH Gradient
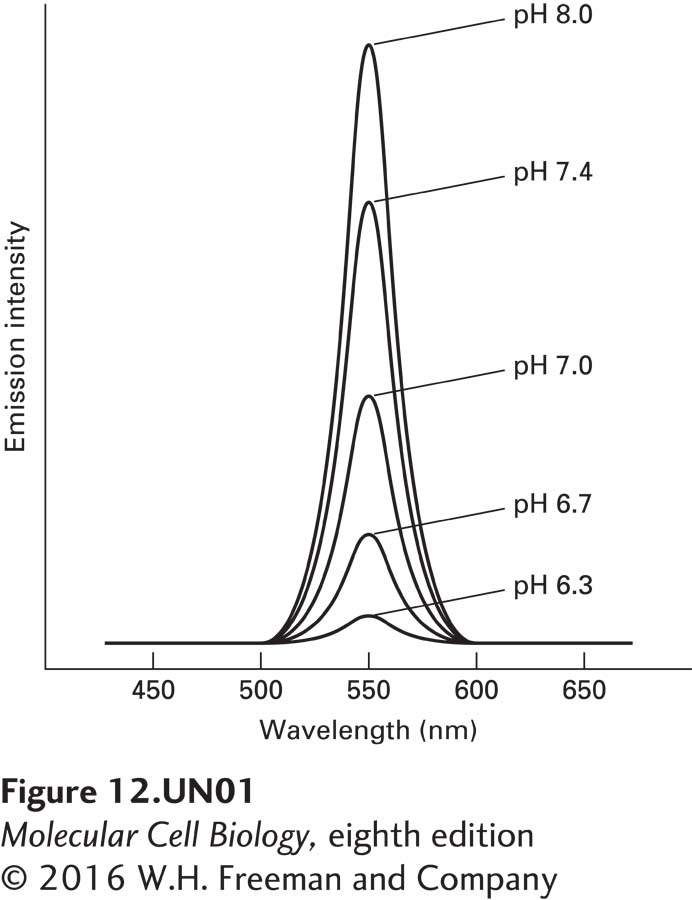
A proton gradient can be analyzed with fluorescent dyes whose emission-intensity profiles depend on pH. One of the most useful dyes for measuring the pH gradient across mitochondrial membranes is the membrane-impermeant, water-soluble fluorophore 2′,7′-bis-(2-carboxyethyl)-5(6)- carboxyfluorescein (BCECF). The effect of pH on the emission intensity of BCECF, excited at 505 nm, is shown in the accompanying figure. In one study, sealed vesicles containing this compound were prepared by mixing unsealed, isolated inner mitochondrial membranes with BCECF; after resealing of the membranes, the vesicles were collected by centrifugation and then resuspended in nonfluorescent medium.
a. When these vesicles were incubated in a physiological buffer containing NADH, ADP, Pi, and O2, the fluorescence of BCECF trapped inside gradually decreased in intensity. What does this decrease in fluorescence intensity suggest about this vesicular preparation?
b. How would you expect the concentrations of ADP, Pi, and O2 to change during the course of the experiment described in part a? Why?
c. After the vesicles were incubated in a buffer containing ADP, Pi, and O2 for a period of time, addition of dinitrophenol caused an increase in BCECF fluorescence. In contrast, addition of valinomycin produced only a small transient effect. Explain these findings.
d. What result would you expect to see if the source of the mitochondrial membranes were brown-fat mitochondria? Explain.
e. Chloroplasts could also be used as a source of membranes in a similar experiment (as in part a) involving BCECF. In this case, the BCECF would be surrounded by what membrane? How would the fluorescence change upon addition of light, ADP, and Pi?
Activity results are being submitted...