
Chapter 13. Juniferdin and PDI
Introduction

Analyze the Data 13-2: Juniferdin and PDI
Recently, researchers discovered that treating mammalian cells with juniferdin, a plant-derived compound, affects protein secretion, and they have reported that the target of this drug is protein disulfide isomerase (PDI). In the following experiment, cultured pancreatic β-cells were treated with juniferdin, and protein lysates were isolated and compared with lysates from untreated cells using immunoblot analysis. Probing of blots with antibodies against PDI (57 kDa), actin (43 kDa), and proinsulin (9.8 kDa) shows the following:
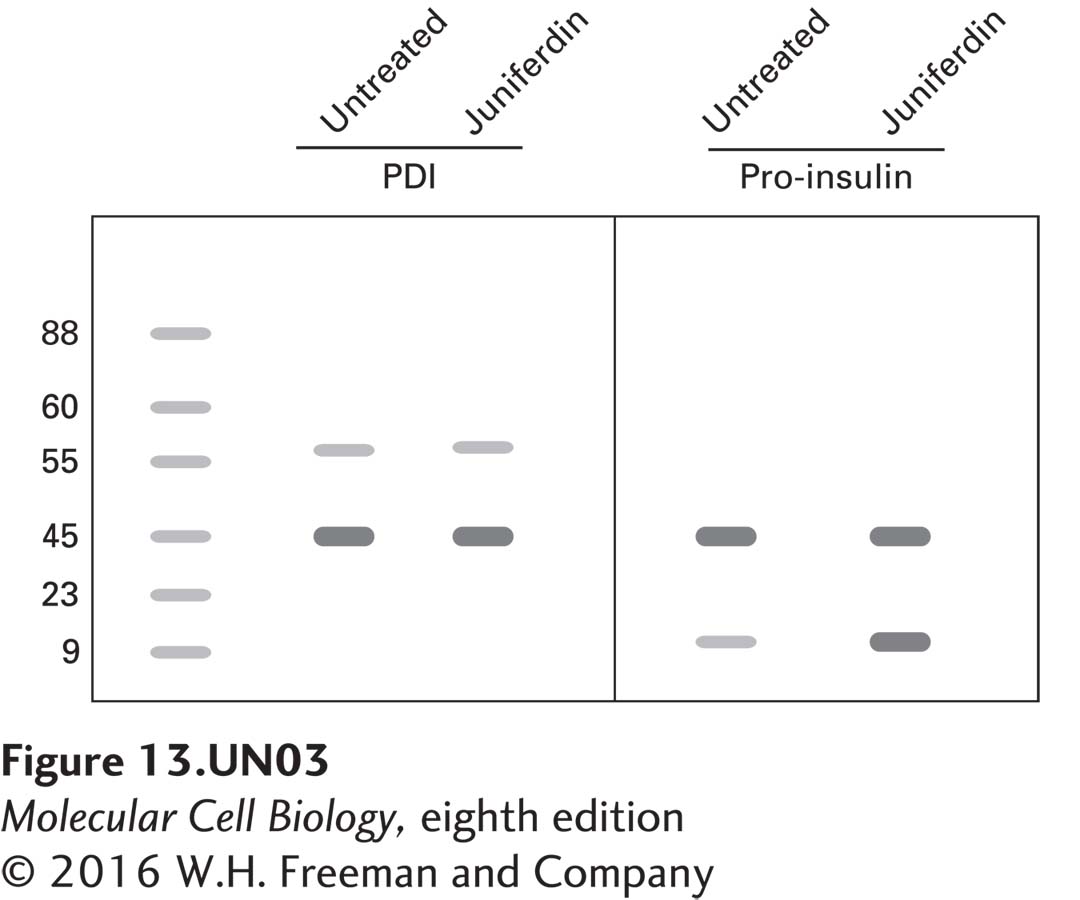
a. Given that approximately the same amount of protein was loaded in each lane, as evidenced by the actin signals, how do you explain the fact that the PDI levels also appear about the same, while most of the proinsulin remains accumulated in the juniferdin-treated cells?
b. To confirm these results, protein lysates of juniferdintreated and untreated cells were separated by SDS-PAGE, blotted to membranes, and then probed with antibodies against Ire1 and Hac1.
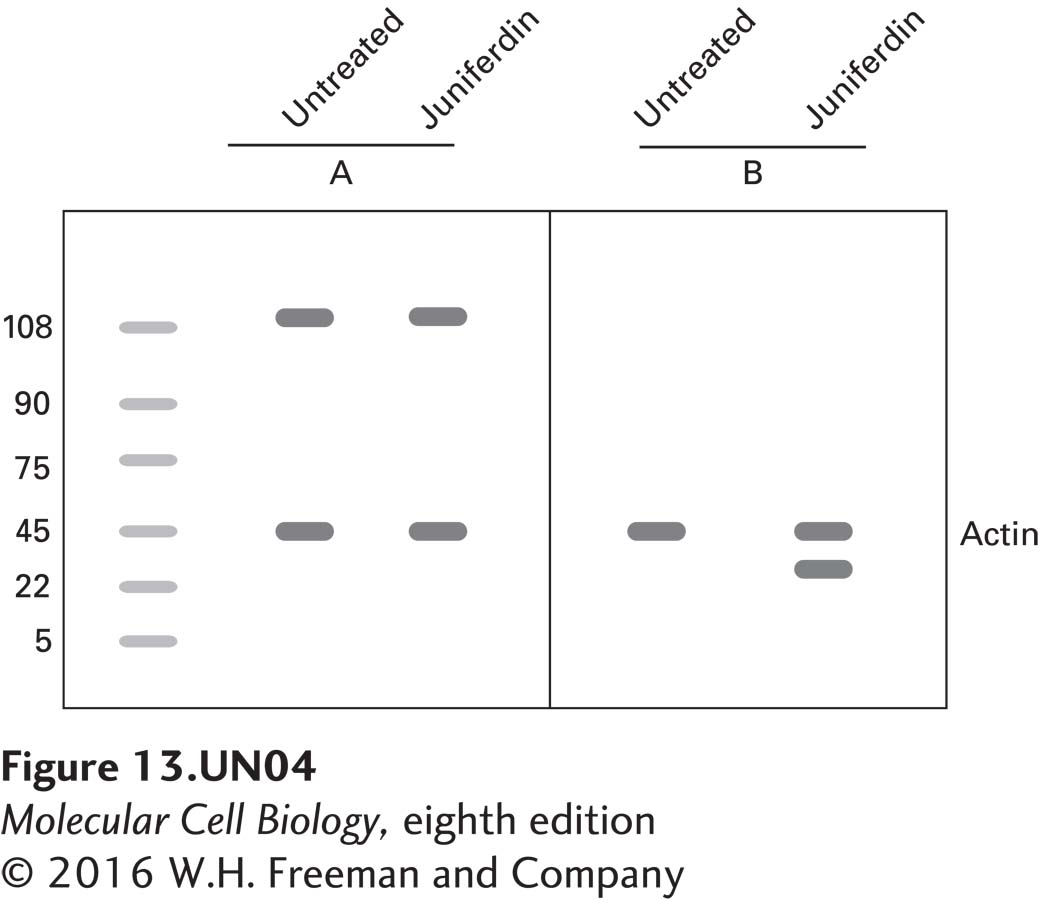
Label each blot with the antibody that was used for the analysis. How do you explain the increase in the intensity of the signal seen in the juniferdin-treated cells in blot B?
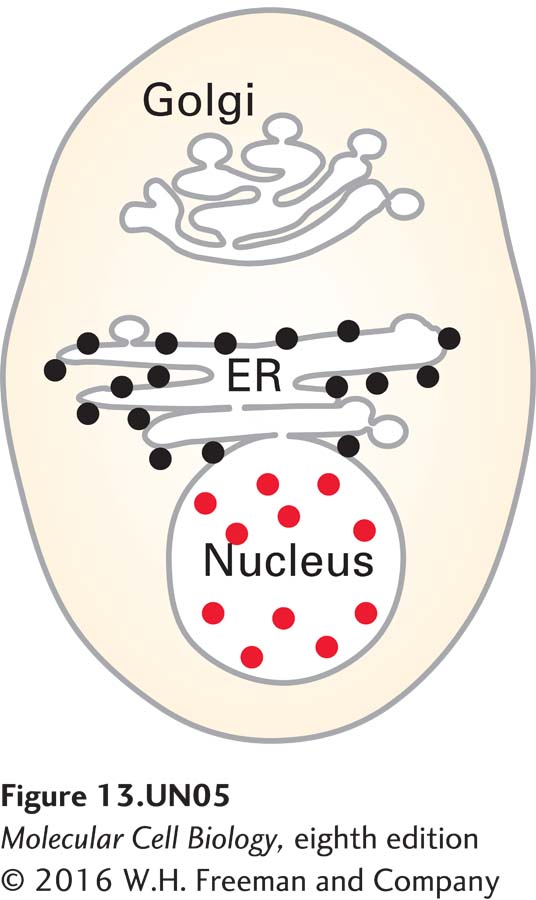
c. An immunocytochemistry and fluorescence microscopy analysis was undertaken with the antibody used in blot B and a secondary antibody labeled with rhodamine (red). Since juniferdin specifically affects PDI, a resident rough ER (RER) protein, how do you explain the localization of the signal in the nucleus?
Activity results are being submitted...