
Chapter 13. Analyzing the Subcellular Localization of Proteins
Introduction

Analyze the Data 13-3: Analyzing the Subcellular Localization of Proteins
Antibody labeling of proteins like that used in immunofluorescence analysis can be applied to electron microscopy, but instead of using fluorescent labels attached to antibodies, investigators use gold particles that are electron dense and appear as uniform dots in an electron micrograph. Furthermore, by varying the size of the gold particles (e.g., 5 nm vs. 10 nm), one can identify the localization of more than one protein in the cell.
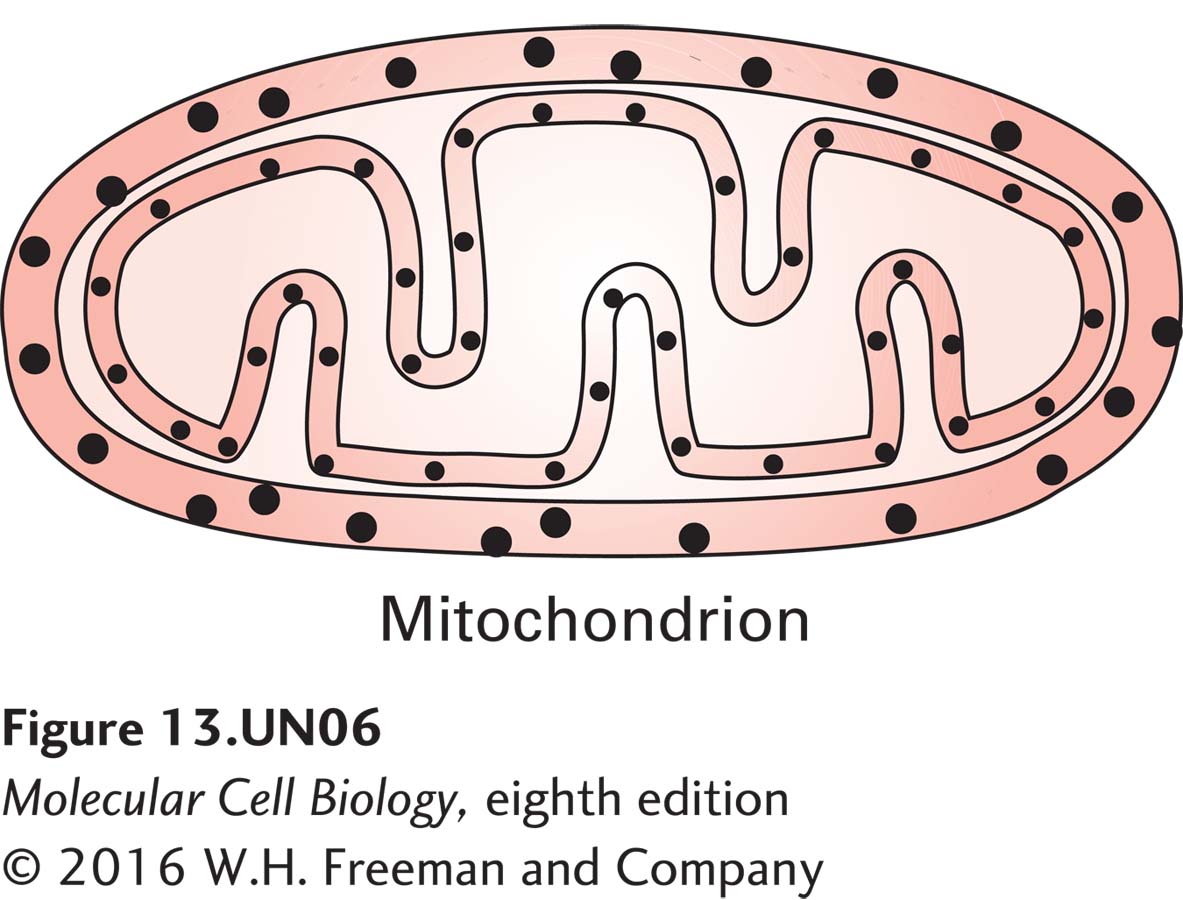
a. Using this approach, investigators have determined the subcellular localization of Tim and Tom proteins used for protein import into mitochondria. In the drawing of the results below, label the gold particles showing the localization of Tim44 and Tom40. What made you come to these conclusions?
b. Using recombinant DNA techniques to fuse the N- terminus of alcohol dehydrogenase (ADH) to a cytosolic protein alters that protein’s localization. The following blot was seen when cells were transfected with an ADH-actin chimeric construct and proteins were isolated from the cytosol and mitochondria. Antibodies against actin (43 kDa), the cytosolic protein GAPDH (37 kDa), and the mitochondrial inner membrane protein succinate dehydrogenase A (72 kDa), were used in the analysis.
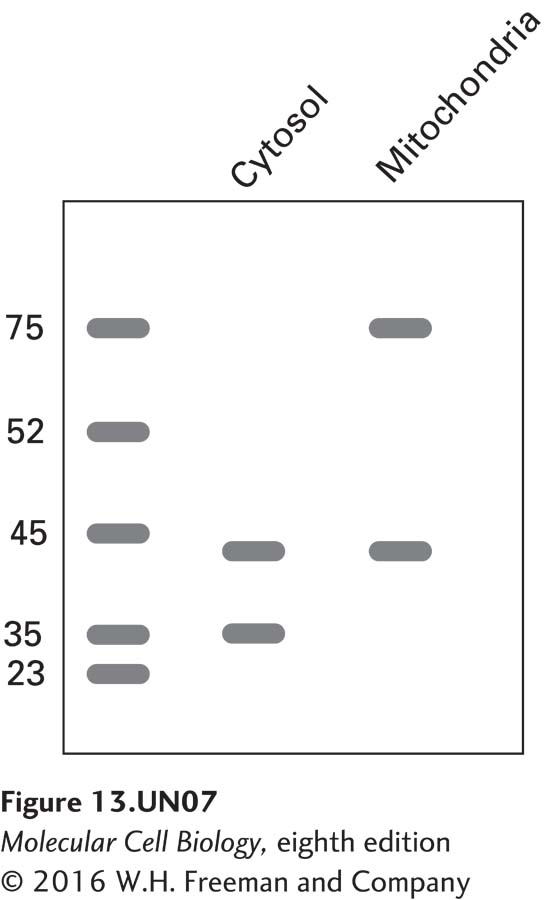
How do you explain the presence of actin in two distinct subcellular pools of protein? How do you explain it being the same molecular mass in both pools? If the blot were stripped and re-probed with an antibody against the N-terminus of alcohol dehydrogenase, where would you expect to find the signal?
c. Cyanide is toxic to cells because it inhibits a specific mitochondrial protein complex that is responsible for producing ATP. An experiment like that described above was repeated and the results compared with those for cells exposed to hydrogen cyanide. The following are the results from the immunoblot analysis:
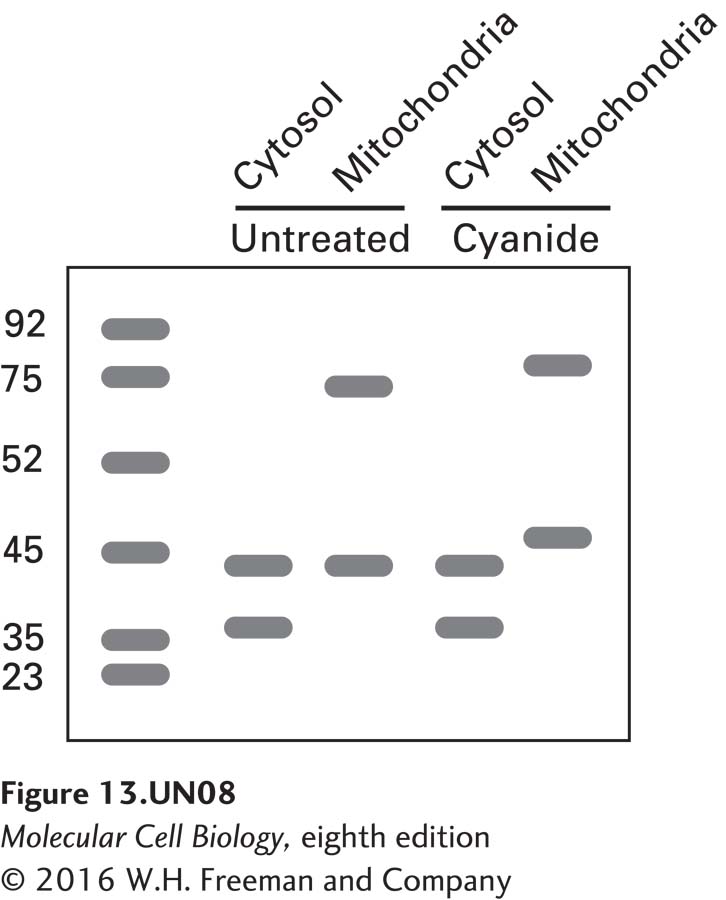
How do you explain the apparent shift in the molecular mass of actin in the mitochondrial fraction of cyanidetreated cells? Note that there is a similar shift in the mass of the succinate dehydrogenase A control. What would you expect to see on blots if cyanide-treated cells were supplemented with ATP?
Activity results are being submitted...