
Chapter 15. Investigating PKA Interactions with FRET
Introduction

Analyze the Data 15-2: Investigating PKA Interactions with FRET
The phosphorylation of a protein can influence its ability to interact with other proteins. These protein-protein interactions play a fundamental role in signal transduction pathways, and these interactions can be identified using numerous techniques, including Förster resonance energy transfer (FRET) (see Figure 15-15). Protein kinase A (PKA) has many substrates, one of which is glycogen phosphorylase kinase (GPK), which has a multimeric (αβγδ)4 structure containing two regulatory subunits (α and β), the catalytic γ subunit, and the calcium sensor δ subunit, calmodulin.
a. You are using FRET to investigate PKA interactions with GPK and have cloned cDNA fusion constructs for three of its four different subunits (γ, β, and δ), all containing a fluorescent tag that when expressed excites at 480 nm and emits fluorescence at 535 nm. You also have cDNA encoding the catalytic domain of PKA fused to a tag that when expressed excites at 440 nm and emits at 480 nm. In the assay, if the PKA fusion protein interacts with one or more of the tagged GPK substrates, the transfer of energy from the PKA tag will excite the tag on the substrate, causing it to emit fluorescence at 535 nm, which can be detected.
Liver cells are transfected with the PKA fusion construct alone (controls) or with the PKA fusion construct plus one of the three tagged GPK constructs. The cells are then treated with epinephrine. The fluorescence emissions at 535 nm resulting from the four different transfection treatments, each averaged over three different repetitions, are shown in the graph below. Label the four bars on the graph, showing the emissions for PKA by itself, PKA + the γ subunit, PKA + the β subunit, and PKA + the δ subunit. Explain why there is only one major peak and why the values represented by the other three bars are not significantly different from one another.
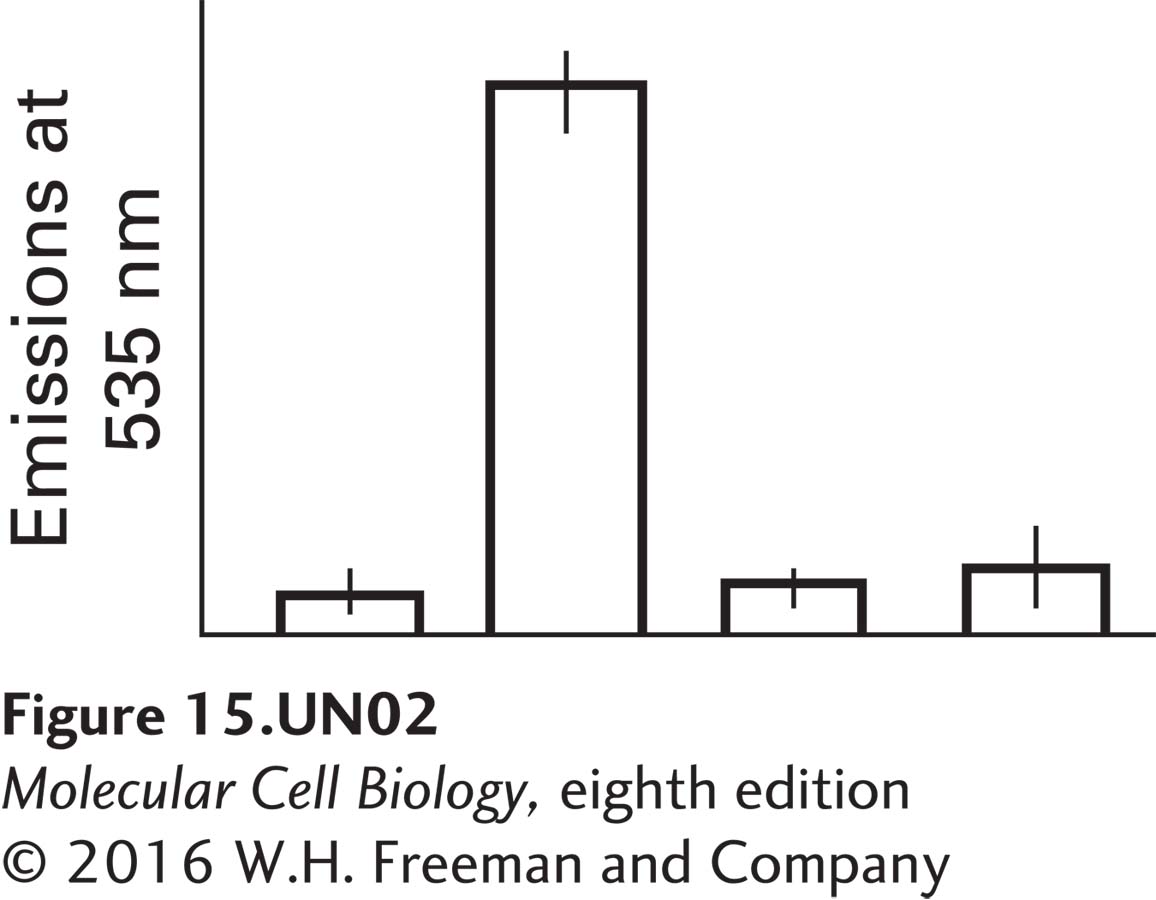
In this case, three of the four bars can be labeled however one likes because there is no FRET. The large peak is due to the PKA interacting with the b subunit, which is one of the two regulatory subunits of glycogen phosphorylase kinase; it is one of the subunits that is phosphorylated by active PKA. There is only one peak because the other subunits of glycogen phosphorylase do not bind to the catalytic domain of PKA.
b. Which combination above would produce emissions at 535 nm if the experiment were repeated, but instead of epinephrine you used dibutyryl-cAMP, which freely crosses the plasma membrane?
The same combination—PKA plus β subunit—would produce emission at 535 nm because even though receptor activation is bypassed, increasing the intracellular cAMP levels would be sufficient to activate PKA.
c. As described above, GPK has two regulatory subunits, both of which are subjected to post-translational modifications. If the gene encoding the α subunit contained missense mutations whereby during translation all the serine, threonine, and tyrosine residues were converted to some other amino acid, how would this affect the calcium sensor subunit of GPK? The activity of GPK in normal cells treated with epinephrine is shown in the following graph.
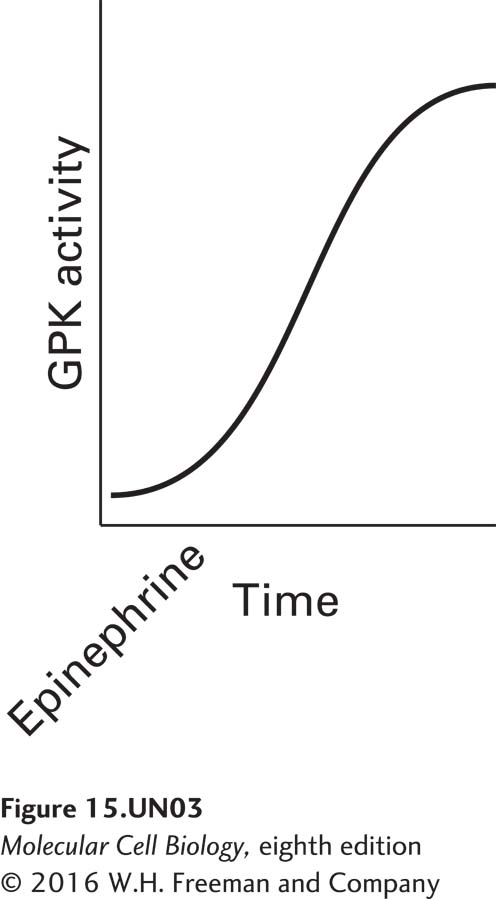
Draw what the activity would look like in comparison in epinephrine-treated cells expressing the α subunit containing the missense mutations described above.
Activity results are being submitted...